Question: What is matter?
Answer: Any substance that has mass and occupies space.
Question: What is matter made up of?
Answer: Matter is made up of very small particles.
Question: What makes liquids take the shape of the container they are poured in?
Answer: Liquids take the shape of the container they are poured in because the particles are not as tightly packed as in solids.
Question: List any three processes to show that matter changes from one state to another.
Answer: The three processes to show that matter changes from one state to another are :-
- Melting
- Freezing
- Evaporation
- Condensation
Question: How does heating and cooling affect the movement of particles?
Answer: On cooling the movement of particles decreases
On heating the movement of particles increases.
Question: Explain in what way is the arrangement of particles related to the states of matter.
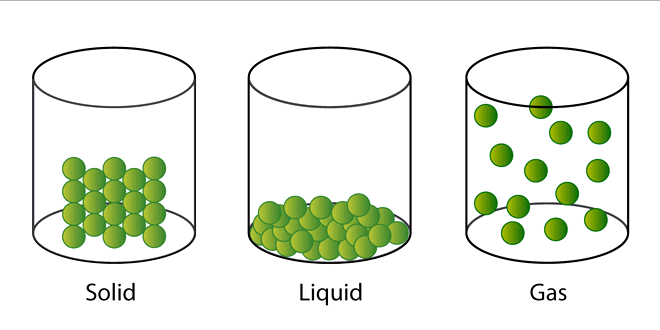
Answer: All matter is made up of every small particles. The arrangement of particles results in three states of matter:-
- Solids: In solids the particles are very closely packed and because of this mostly solids have fixed shape.
- Liquids: In liquids particles are not as closely packed as in solids. This is the reason liquids can take the shape of the container they are poured into.
- Gases: In gases particles are very loosely packed and they can easily flow from one place to another.
Question: Describe how the states of matter change in terms of the movements of tiny particles.
Answer: In terms of the movement of particles the changes in states of matter will be as follow:
- Melting: In this process a solid changes into a liquid on heating. The particles of the solid will start changes moving faster on heating and break away from their rigid pattern thus making a liquid.
- Freezing: In this process a liquid changes into a solid on cooling. When the liquid is cooled the movement of its particles will decrease and they will pack themselves into a rigid shape thus forming a solid.
- Evaporation: In this process a liquid changes into a gas on heating. The particles start moving faster on heating and break free from their existing pattern thus making a gas.
- Condensation: In this process a gas changes into a liquid on cooling. Cooling of the gas will decrease the movement of its particles making them free to move thus forming a liquid.
Question: Explain the processes of contraction and expansion.
Answer: The processes of contraction and expansion
Expansion:
- In this process the size of the matter increases.
- When the substance is heated the particles start moving rapidly
- Because of the increased movement of particles they take up more space and thus expand.
Contraction:
- In this process the size of the matter decreases.
- When the substance is cooled the movement of particles slows down.
- Because of the decreased movement of particles they take up less space and thus contract.
Question: Describe an example to show that substances expand on heating and contract on cooling.
Answer: Due to expansion and contraction the electric cables appear to hang loosely from the poles during summer but do not appear loose in winter.
Question: Differentiate between solids, gases and liquids.
Answer:
Question: Name some liquids which evaporates easily.
Answer: Liquids which evaporates easily are :-
Alcohol, kerosene, petrol, water and nail paint remover alcohol.
Question: Rita want’s to get ready for party but her dress is wet. Suggest some ways by which she can dry her dress.
Answer: Ways by which she can dry her dress are :-
- If it is morning time she can let her dress dry under the sun.
- She can iron the dress.
- She can dry the dress using a hair dryer.
Question: Why do clothes dry faster in summers than in winters.
Answer: Due to the heat of sun and the temperature is high in summers that is why clothes dry faster in summers than in winters.
Question: Why do chocolates melt on heating?
Answer: Chocolates melt on heating because when the particles are heated they break the rigid pattern and start moving faster thus taking space than before.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students


Very good website, it is very helpful for my exams!
Very good website, it is very helpful for my final exams