Acids Bases and Salts 7th Class NCERT CBSE Science Chapter 05
Question: What is the test for acids and bases using litmus paper?
Answer: Acids turn blue litmus paper red while bases turn red litmus paper blue.
Question: What name is given to those substances which do not change the colour of either red or blue litmus paper?
Answer: The solutions or substances which do not change the colour of either red or blue litmus paper are neutral.
Question: Write the name of two indicators which occur naturally.
Answer:
- Turmeric
- China rose
Question: Name the acid present in lemon juice and tomato.
Answer: Citric acid in lemon juice and oxalic acid in tomato.
Question: Name a substance which is used to cure the discomfort caused by indigestion due to over eating.
Answer: Milk of magnesia which contains magnesium hydroxide (base) is used to neutralize the effect of excessive acid in stomach.
Question: Why does a turmeric stain on white shirt is turned to red when it is washed with soap?
Answer: Turmeric stain on white shirt is turned to red when it is washed with soap because the soap solution is basic in nature.
Acids Bases and Salts – Question: Is lime water an acidic or basic solution?
Answer: Lime water (calcium hydroxide) is a basic solution.
Question: Name the reaction when an acid is mixed with base.
Answer: Neutralization reaction.
Question: Which acid is injected into our body by an ant sting that causes pain?
Answer: The acid is formic acid which is injected into the skin of a person when an ant bites.
Question: Name the salt formed when hydrochloric acid reacts with sodium hydroxide solution (a base).
Answer: When hydrochloric acid reacts with sodium hydroxide solution, then sodium chloride salt is formed.
Question: Write two basic substances which we use in our daily life.
Answer:
- Baking soda
- Soap or detergent
Acids Bases and Salts – Question: Name one acid which is responsible for acid rain.
Answer: Carbonic acid.
Question: Name two gaseous pollutants which are responsible for acid rain.
Answer: Two gaseous pollutants are nitrogen dioxide (NO2) and sulphur dioxide (SO2).
Question:
- Name one indicator which turns red on adding an acid.
- Name one indicator which turns red on adding a base.
Answer:
- Litmus (blue litmus paper)
- Turmeric
Question: Form a sentence using the following words: baking soda, ant bite, moist, effect, neutralized, rubbing.
Answer: The effect of an ant bite can be neutralized by rubbing moist baking soda.
Question: Write the characteristics of acids by which we can identify acids.
Answer: Characteristics of acids are
- They are sour in taste.
- They turn blue litmus paper / solution to red.
- Dilution of acid in water is an exothermic reaction, i.e. heat energy is evolved.
Question: While playing in a park, a child was stung by a wasp. Some elders suggested applying paste of baking soda and others lemon juice as remedy. Which remedy do you think is appropriate and why?
Answer: Wasp sting inject a liquid in the skin which is acidic in nature. Hence, baking soda is the appropriate remedy as it is basic in nature and neutralizes the acid.
Acids Bases and Salts – Question: Which of the following are acidic and which are basic?
Lime water, Vinegar, Toothpaste, Stomach juices, Lemon juice, Baking soda solution, Milk of magnesia, Ammonia solution.
Answer:
| Acidic in nature | Basic in nature |
| Vinegar | Lime water |
| Stomach juices | Toothpaste |
| Lemon juice | Baking soda solution |
| Milk of magnesia | |
| Ammonia solution |
Question: A small amount of hydrochloric acid is always produced in the stomach. Is it useful or harmful for us? If excess of acid is produced in the stomach, what should we do?
Answer: A small amount of hydrochloric acid produced in the stomach is useful as it can kill the harmful bacteria that may enter into the stomach along with the food.
However, if excess of acid is produced, there is a burning sensation in the stomach. We should take milk of magnesia as an antacid medicine to neutralize the excess acid.
Question: Write the effect of China rose petals on acidic and basic solutions.
Answer: China rose petals when added to warm water, form a light pink colored solution which may be used as an indicator. This indicator turns acidic solution to magenta (deep pink) and basic solution to green.
Question: To test the presence of an acid in any substance, what methods can be used?
Answer: Acid can be tested in any substance by the following methods
- Take a drop of the dilute solution of the substance on the tips of your tongue. If tastes sour, it is acidic.
- Dip a blue litmus paper in the solution. If the colour of the litmus paper turns red, the solution is acidic.
Question: Why are sodium bicarbonate and lemon juice used during indigestion?
Answer: Sodium bicarbonate neutralizes the acidity in the stomach. Hence, it is used during indigestion. Lemon contains acid. It reacts with undigested food and suffers it.
Question: After carrying out the neutralization reaction, the test tube immediately found to be somewhat hot. Explain why.
Answer: In neutralization reaction, heat is always produced or evolved. The evolved heat raises the temperature of the reaction mixture. Therefore, if we touch the test tube immediately after the neutralization reaction, it is found to be hot.
Acid + Base → Salt + Water (heat is evolved)
Acids Bases and Salts – Question: Name three acids used in the laboratory.
Answer: Acids which are mostly used in laboratory as below
- Hydrochloric acid (HCl)
- Sulphuric acid (H2SO4)
- Nitric acid (HNO3)
Question: Paheli is suffering from indigestion due to acidity. Is it advisable to give her orange juice in this situation and why?
Answer: No, because orange juice is acidic in nature. Excess of acid in the stomach causes indigestion. We take an antacid such as milk of magnesia which contains magnesium hydroxide.
Question: Explain two neutralization reactions related to daily life situations.
Answer:
- Ant bite When an ant bite injects the acidic liquid (formic acid) into the skin, the effect of the acid can be neutralized by rubbing moist baking soda (sodium hydrogen carbonate) or calamine solution, which contains zinc carbonate.
- Indigestion Our stomach contains hydrochloric acid. It helps us to digest food but too much of acid in the stomach causes indigestion. Sometimes, indigestion is painful. To relieve indigestion, we take an antacid such as milk of magnesia which contains magnesium hydroxide. It neutralizes the effect of excessive acid.
Question: How lime water is prepared in the laboratory?
Answer: To prepare lime water, dissolve some lime (chuna) in water in a bottle. Stir the solution and keep it for sometime. Pour a little more from the top. This is lime water.
Question: Name three types of salts. Give one example of each type of salt.
Answer: Type of salts
- Neutral salts e.g. sodium chloride (NaCl)
- Acidic salts e.g. Ammonium chloride (NH4Cl)
- Basic salts e.g. sodium hydrogen carbonate (NaHCO3)
Question: Paheli observed that most of the fish in the pond of her village were gradually dying. She also observed that the wastes of a factory in their village are flowing into the pond which probably caused the fish to die.
- Explain why the fish were dying?
- If the factory waste is acidic in nature, how can it be neutralized?
Answer:
- If the wastes of a factory flow into waterbodies, it can cause a threat to the lives of sea creatures and to anybody who drink the water. Since, factory wastes may contain acids or bases and it can kill the fish.
- If the factory waste is acidic in nature, it can be neutralized by adding basic substances.
Question: What is a salt? Name any salt and give their formulae.
Answer: A substance formed by the neutralization of an acid with a base is called salt. Salt may be acidic, basic or neutral in nature.
For example:
Hydrochloric acid (HCI) +
(Acid)
Sodium hydroxide (NaOH) →
(Base)
Sodium chloride (NaCI) + Water (Salt)
(Salt)
Question: Look at the given reaction.
Hydrochloric acid + Sodium hydroxide (base) → Sodium chloride (salt) + Water
Sodium chloride formed in this reaction remains in solution form. Can we get solid sodium chloride from this solution? Suggest a method (if any).
Answer: We can get solid sodium chloride by evaporation method. Evaporation is the process by which water changes from a liquid to a gas or vapour.
Rate of evaporation increases with temperature.
Acids Bases and Salts – Question: Name three bases used in the laboratory with their formulae.
Answer: Bases which are mostly used in laboratory as below:
- Sodium hydroxide (NaOH)
- Calcium hydroxide [Ca(OH)2]
- Ammonium hydroxide (NH4OH)
Question: A farmer was unhappy because of his low crop yield. He discussed the problem with an agricultural scientist and realized that the soil of his field was either too acidic or too basic. What remedy would you suggest the farmer to neutralize the soil?
Answer: The reason for low crop yield in farmer’s field was that the soil at a place is either too acidic or too basic. The soil may be acidic or basic naturally. The excessive use of fertilizers in the fields also makes the soil too acidic. When the soil is too acidic, it is treated with base such as quicklime (calcium oxide) or slaked lime (calcium hydroxide) which neutralizes the excess acid present in the soil and reduces its acidic nature. If the soil is too basic, then decaying organic matter (called manure or compost) is added it. The decaying organic matter releases acid which neutralizes the excess bases present in the soil and reduces its basic nature.
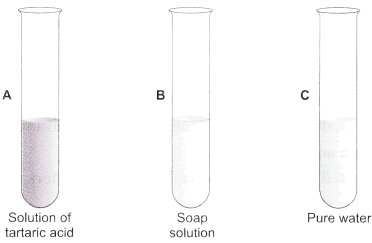
Question: You are provided with three test tubes A, B and C as shown in figure with different liquids. What will you observe when you put
- a piece of blue litmus paper in each test tube?
- a piece of red litmus paper in each test tube?
- a few drops of phenolphthalein solution to each test tube?
Answer:
| Test tube | Effect on blue litmus paper | Effect on red litmus paper | Effect on phenolphthalein solution |
| A | Turns red | Remains red | Colorless |
| B | Remains blue | Turns blue | Pink colour |
| C | Remains blue | Remains red | Colorless |
Question: Boojho, Paheli and their friend Golu were provided with a test, each containing China rose solution which was pink in colour. Boojho added 2 drops of solution ‘A’ in his test tube and got dark pink colour. Paheli added 2 drops of solution ‘S’to her test tube and got green colour. Golu added 2 drops of solution ‘C’ but could not get any change in colour. Suggest the possible cause for the variation in their results.
Answer:
China rose (gudhal) is an acid-base indicator.
China rose solution in different medium shows following changes:
China rose + acid → Give dark pink colour, hence A is an acidic solution.
China rose + base → give green colour, hence 6 is a basic solution.
China rose + neutral → No change in colour, hence C is a neutral solution.
Question: Nitesh was playing with his friends in the garden. Suddenly, Nitesh was stung by a honeybee and was in great pain. Immediately, his friends call his mother. She applied baking soda solution on the affected area and then took him to the doctor.
Read the above passage and answer the following questions:
- What could be the reason for this burning pain?
- Why did his mother applied baking soda solution on the affected area?
- What values are shown by Nitesh’s friends? [Value Based Question]
Answer:
- The reason for this burning pain is honeybee sting which causes pain and irritation. This is due to the acidic liquid (formic acid) injected into the skin by the honeybee.
- The effect of acid can be neutralized by rubbing the affected area by baking soda solution which is a mild base.
- Nitesh’s friends are caring , supportive and helpful.
Question: One day Rahul’s mother after taking meal felt pain and irritation in her stomach. His father was out of station. Rahul was an intelligent boy. He remembered his teacher’s statement and gave his mother some baking soda solution Which gave her a relief from pain and irritation of stomach.
Read the above passage and answer the following questions.
- Which information given by Rahul’s teacher that helped him to select the baking soda as remedy?
- Why he selects baking soda as a cure?
- What values are shown by Rahul?
Answer:
- Rahul knows that our stomach contains hydrochloric acid. It helps us to digest food. But, too much acid in the stomach causes indigestion. Sometimes, indigestion is painful and some mild base should be taken to relief from this pain. So, this information given by his teacher helped him.
- He gave her mother baking soda solution which was available in the kitchen easily. He selects baking soda because it is a mild base and neutralizes the excess acid in the stomach and it will give relief from pain.
- Rahul is very intelligent and caring boy.
Fill in the Blanks: Acids Bases and Salts
- Lemon juice and vinegar taste ……………… because they contain ……………… .
- Turmeric and litmus are ……………… acid-base indicators.
- Phenolphthalein gives ……………… colour with lime water.
- When an acidic solution is mixed with a basic solution, they ……………… each other forming ……………… and water. [NCERT Exemplar]
- The acidic or basic nature of a substance is tested by using an ……………… .
- The substances which show different colors in acidic, basic and neutral solutions are called ………………
- Acid + Base → ……………… + Water
- When an acid mixed with a base, a ……………… reaction takes place.
- Acetic acid is present in ……………… while ……………… is present in lemon.
- Excessive use of chemical fertilizers make the soil ……………… .
- Hydrochloric acid + Sodium hydroxide → ……………… + Water
- Acids turn blue litmus ……………… .
Answer:
- sour, acids
- natural
- pink
- neutralize, salt
- indicator
- indicators
- salt
- neutralization
- vinegar, citric acid
- acidic
- Sodium chloride
- red
True / False
- All substances are either acidic or basic.
- A compound if acidic will turn all indicators red.
- Lime water turns red litmus blue.
- Common salt dissolved in water turns blue litmus red.
- Phenolphthalein is a natural indicator.
- Calamine can be used to treat ant’s sting.
- Lemon water is basic in nature.
Answer:
- False, substances can be neutral as well.
- False, acids do not turn all indicators red.
- True
- False, it does not change the colour of litmus at all.
- False, it is a man-made indicator.
- True
- False, it is acidic in nature.
Question: Match the substances in Column I with those in Column II.
| Column I | Column II |
| (a) Tartaric acid | (i) Soap |
| (b) Calcium hydroxide | (ii) Curd |
| (c) Formic acid | (iii) Unripe mangoes |
| (d) Sodium hydroxide | (iv) Ant’s sting |
| (e) Lactic acid | (v) Lime water |
Answer:
(a) – (iii)
(b) – (v)
(c) – (iv)
(d) – (i)
(e) – (ii)
Acids Bases and Salts – Question: Match the items in Column 1 with Column II.
| Column I | Column II |
| (a) Vinegar | (i) is used as indicator |
| (b) Sodium chloride | (ii) is sour to taste |
| (c) Milk of magnesia | (iii) major salt of sea |
| (d) Turmeric | (iv) changes red litmus blue |
Answer:
(a) – (ii)
(b) – (iii)
(c) – (iv)
(d) – (i)
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students