Question: What is an acid-base indicator or indicator? Explain in brief about the various types of natural indicators.
Answer: Substances that assist in determining whether a given substance is acidic or basic, with the help of a colour change, are called indicators. The will not effect the nature of the solution to be tested and a very little quantity of indicator is sufficient to observe the colour change. Turmeric, China rose and litmus are some of the naturally used indicators. Phenolphthalein, litmus and mythyl orange are used commonly in laboratories.
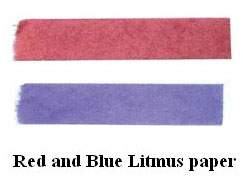
Litmus: Litmus is the most commonly used natural indicator extracted from lichens. Two types of litmus are available, in the form of a solution, referred to as ‘Litmus Solution’ and as strips of paper called ‘Litmus Paper’. If acidic substances are added to blue litmus, it turns red, while red litmus turns blue, when basic substances are added to it.
Turmeric: Turmeric (Haldi) that is used in kitchen can also be used as one of the natural indicators for bases. In acidic and neutral substances, the turmeric solution remains yellow in colour, while in basic substances, it turns red in colour.
China rose: China rose (Hibiscus) juice obtained from the petals is used as a natural indicator. If acidic substances are added, it turns dark pink (magenta) in colour, while adding basic substances makes the juice green.
Question: Write properties of a base.
Answer: The properties are:
- Bases are bitter to taste.
- Bases turn red litmus to blue.
- Bases are slippery to touch.
- Some bases are soluble in water, they are called alkalis. Some are not soluble in water.
- Bases are corrosive in nature, so they should not be touched with bare hands.
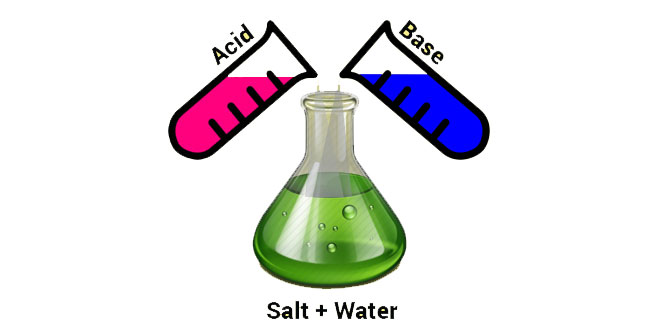
- Base react with acids to form salt and water.
- Base react with metals to form salt and hydrogen gas.
Question: Write the properties of Acid.
Answer: The properties are:
- Acids are sour to taste.
- Acids turn blue litmus to red.
- Acids can corrode metals like aluminum and iron due to their corrosive nature. That is why acids are stored in glass containers.
- Most of the mineral acids cause burns, so care should be taken while handling these acids.
- Acids are soluble in water.
- Acids react with metals to form salt and hydrogen gas.
- Acids react with bases to form salt and water.
- Acids react with carbonates to form salt and carbon dioxide gas.
Question: Give two main uses of each: Sulphuric acid, hydrochloric acid, Nitric acid.
Answer: Two main uses are:
Sulphuric Acid: Its main uses are-
- To manufacture fertilizers such as ammonium sulphate and superphosphate.
- They are used in automobile batteries.
Hydrochloric Acid: Its main uses are-
- It removes scales, i.e. the deposits formed inside boiler in various industries. The process is called descaling.
- To make aqua regia.
Nitric Acid: Its main uses are-
- It is used to prepare Aqua Regia.
- It is used to purify precious metals such as silver and gold.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students


Such a nice app and I have learnt a lot of thing from it.