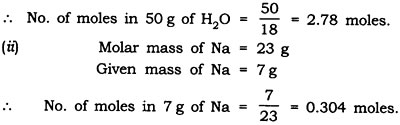Question: Showing results for the molecular formula of glucose is C6H12O6. Calculate its molecular mass. (Atomic masses: C = 12 u; H = 1 u; O = 16 u)
Answer: It is very simple to calculate molecular weight of any compound when you know atomic Number of elements.
Glucose = C6H12O6
C (atomic Number)=6
H (atomic Number)=1
O (atomic Number)=8
Multiply by 2 in each elements then you will get molecules weight of each elements
C=6×2=12
H=1×1=1
O=8×2=16
Then multiply by Number of elements by molecules weight of C, H and O
C6H12O6 = C(6×12)+H(12×1)+O(6×16)=180 Molecules weight of glucose
This is the simple method to calculate molecular weight of any compound if you know atomic Number of elements and just multiply by 2 in atomic Number you will get molecules weight
Question: Find the number of moles in the following:
- 50 g of H2O
- 7 g of Na
Answer: Number of moles in
- Molar mass of H2O = 18 g
Given mass of H2O = 50 g
Question: Find the number of atoms in the following:
- 0.5 mole of C atom
- 2 mole of N atom
Answer:
- 0.5 mole of C atom:
Number of atoms in 1 mole of C atom = 6.022 x 1023 atoms
Number of atoms in 0.5 mole of C atom = 6.022 x 1023 x 0.5
= 3.011 x 1023 atoms - 2 mole of N atom:
Number of atoms in 1 mole of N atom = 6.022 x 1023 atoms
Number of atoms in 2 mole of N atom = 6.022 x 2 x 1023
= 1.2044 x 1024 atoms
Question: Find the mass of the following:
- 6.022 x 1023 number of O2 molecules
- 1.5 mole of CO2 molecule
Answer:
- 6.022 x 1023 number of 02 molecules:
Mass of 1 mole of O2 molecule = 6.022 x 1023 molecules = 32 g - 1.5 mole of CO2 molecule:
Mass of 1 mole of C02 molecule = 6.022 x 1023 molecules = 44 g
Mass of 1.5 mole C02 molecule = 44 x 1.5 = 66 g
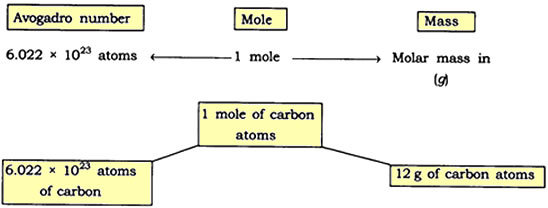
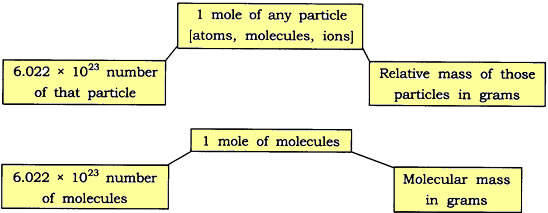
Question: Show the relationship between mole, Avogadro number and mass.
Answer:
Question: What are the rules for writing the symbol of an element?
Answer: IUPAC —> International Union of Pure and Applied Chemistry approves name of elements.
Symbols are the first one or two letters of the element’s name in English. The first letter of a symbol is always written as a capital letter (upper case) and the second letter as a small letter (lower case).
e.g., Hydrogen —> H Helium —> He
Some symbols are taken from the names of elements in Latin, German or Greek.
e.g., Symbol of iron is Fe, its Latin name is Ferrum.
Symbol of sodium is Na, its Latin name is Natrium.
Question: Explain relative atomic mass and relative molecular mass.
Answer: Relative atomic mass: It can be defined as the number of times one atom of given element is heavier than 1/12 th of the mass of an atom of carbon-12.
Relative Molecular Mass: It is defined as the number of times one molecule of a substance or given element is heavier than 1/12 th of the mass of one atom of carbon-12.
Question: The formula of carbon-dioxide is CO2. What information do you get from this formula?
Answer:
- CO2 represents carbon-dioxide.
- CO2 is one molecule of carbon-dioxide.
- CO2 is one mole of carbon-dioxide i.e., it contains 6.022 x 1023 molecules of carbon dioxide.
- CO2 contains 1 atom of carbon and two atoms of oxygen.
- CO2 represents 44 g of molar mass.
Question: State 3 points of difference between an atom and an ion.
Answer:
Atom:
- An atom has no charge.
- Number of electrons = number of protons.
- Atom is reactive.
Ion:
- An ion has either positive or negative charge.
- Number of electrons ≠ number of protons.
- Ion is stable.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students