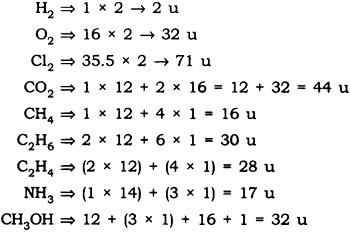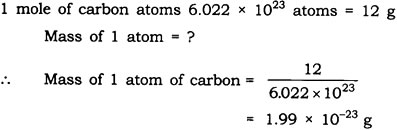Question: Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Answer: The relative number and kinds of atoms are constant in a given compound.
Question: Define the atomic mass unit.
Answer: One atomic mass unit is equal to exactly one-twelfth (1/12th) the mass of one atom of carbon-12. The relative atomic masses of all elements have been found with respect to an atom of carbon-12.
Question: Why is it not possible to see an atom with naked eyes?
Answer: Atom is too small to be seen with naked eyes. It is measured in nanometres.
1 m = 109 nm
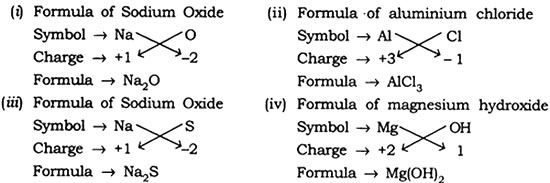
Question: Write down the formulae of
(i) Sodium oxide
(ii) Aluminium chloride
(iii) Sodium sulphide
(iv) Magnesium hydroxide
Answer: The formulae are
Question: What is meant by the term chemical formula?
Answer: The chemical formula of the compound is a symbolic representation of its composition, e.g., chemical formula of sodium chloride is NaCl.
Question: How many atoms are present in a
- H2S molecule and
- P043- ion?
Answer:
- H2S —> 3 atoms are present
- P043- —> 5 atoms are present
Question: Calculate the molecular masses of H2, O2, Cl2, C02, CH4, C2H2,NH3, CH3OH.
Answer: The molecular masses are:
Question:Calculate the formula unit masses of ZnO, Na2O, K2C03, given atomic masses of Zn = 65 u, Na = 23 u, K = 39 u, C = 12 u, and O = 16 u.
Answer: The formula unit mass of
- ZnO = 65 u + 16 u = 81 u
- Na2O = (23 u x 2) + 16 u = 46 u + 16 u = 62 u
- K2C03 = (39 u x 2) + 12 u + 16 u x 3
= 78 u + 12 u + 48 u = 138 u
Question: If one mole of carbon atoms weigh 12 grams, what is the mass (in grams) of 1 atom of carbon?
Answer:
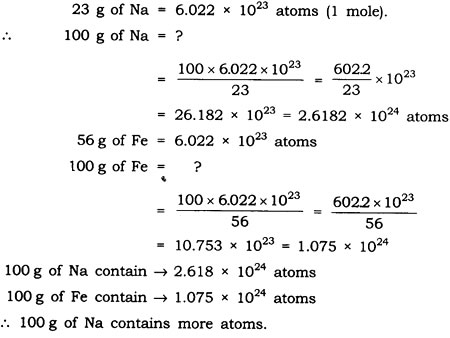
Question: Which has more number of atoms, 100 grams of sodium or 100 grams of iron (given atomic mass of Na = 23 u, Fe = 56 u)?
Answer:
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students