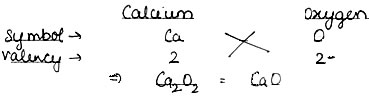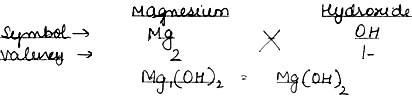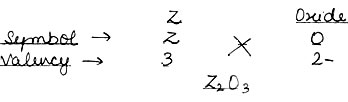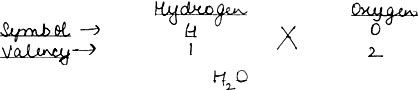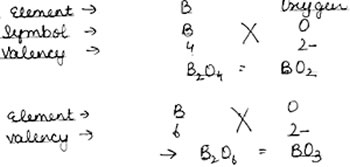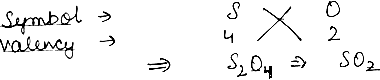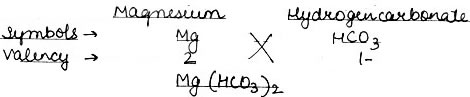Question: State the law of conservation of mass give one example to illustrate this law.
Answer: The law of conservation of mass states that in a closed system, the mass of the system cannot change over time. Look at our example of the candle in the closed room. Though much of the wax itself is no longer present in its original form, all of the mass of the wax is still present in the room, albeit in a different form.
When the flame was lit, oxygen gas from the room reacted with the candle wax to produce water vapor and carbon dioxide gas. If you massed the reactants oxygen and wax, it would equal the mass of the products water and carbon dioxide. We can remember the law of conservation of mass with this simple statement: The mass of the reactants must equal the mass of the products.
Sadly for fans of magic, anything that has mass, including matter and energy, cannot be created or destroyed. That means, mass cannot simply appear out of nowhere and equally it cannot disappear. Matter may change forms however, giving the illusion of nothing out of something or vice versa, but the mass of the matter is always the same before and after the change. If 22 grams of reactants go into a chemical reaction, then 22 grams of products must be produced.
Eg: Calcium Carbonate (100g) → Calcium Oxide (56 g) + Carbon Dioxide (44 g ) = (100 g)
Question: State the law of constant proportions. Gibe one example.
Answer: The law of constant proportions states that a chemical compound always consists of the same elements combined together in the same proportion by mass.
Eg:- Water is a compound which always consists of the same two elements Hydrogen & Oxygen in the fixed ratio – 1:8.
Question: (1) What is the significance of the of an elements? Explain with Examples.
(2) Explain the significance of H.
Answer:
- A’symbol’ is a thing which represents something else. They are use for simplicity. The symbols of elements help us write the element’s name in short. This saves time and is convent. It is 1st and another latter of an element.
- The symbol H represents the element Hydrogen.
Question:
Answer: Atoms of most of the elements exist in the form of molecule or ion, since they are most reactive. For example, hydrogen, oxygen, chlorine, etc. However, atoms of some elements, which are non-reactive, exist in free-state in nature. For example helium, neon, argon, etc.
Usually atoms exist in following two forms:
- In the form of molecules
- In the form of ions
Question: What is a molecule? Explain with example.
Answer: Molecule:
- Molecule is the smallest particle of a compound.
- Atoms exist in free states in the form of molecule.
- Molecule may be formed by the combination of two or more similar atoms of an element, such asoxygen molecule is formed by the combination of two oxygen atoms, molecule of hydrogen which is formed by the combination of two hydrogen atoms.
- Molecules may be formed by the combination of atoms of two or more different elements. For example molecule of water. It is formed by the combination of two atoms of hydrogen and one atom of oxygen. Molecule of Nitric oxide or nitrogen monoxide. It is formed by the combination of one nitrogen atom and one oxygen atom.
- A molecule takes part in chemical reaction.
- Most of the atoms exist in the form of molecule. Molecules are formed by the combination of two or more elements.
Example: Molecule of hydrogen (H2), Molecule of oxygen (O2), Molecule of nitrogen (N2), etc.
Question: What is the different between molecule of an element and molecule of a compound?
Answer: The molecule of an element contains two (or more) similar atoms chemically combined together. Eg:- H2, N2, O2 etc.
Question: Write down formula: (1) Calcium Oxide and(2) Magnesium Hydroxide.
Answer: (1). Calcium Oxide:
(2) Magnesium Hydroxide:
Question: An element Z has a valency of 3. What is the formula of oxide of Z.
Answer:
Question: Name the element water is made of. What are the valencies of these elements? Work out the chemical formula of water.
Answer: Water is made up of two elements → Hydrogen & Oxygen.
Question: An element B shows valencies of 4 and 6.Write the formula of its oxides.
Answer:
Question: Work out the formula Sulphur Dioxide.
Answer:
Question: Work out magnesium hydrogen carbonate.
Answer:
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students