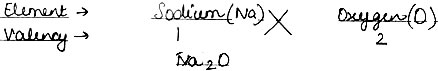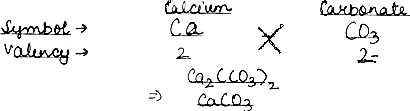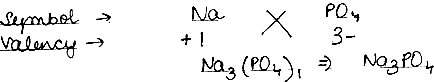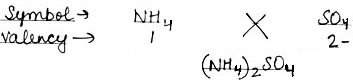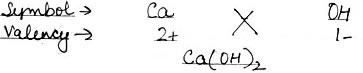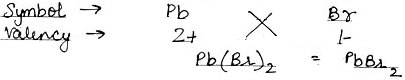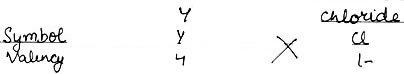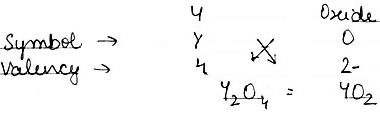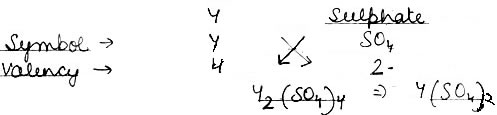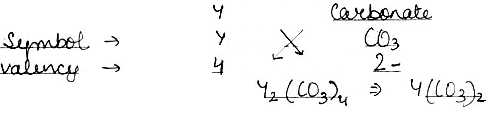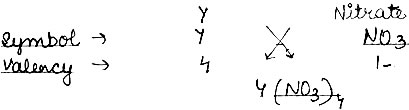Question: Work out the formula: (1) Sodium Oxide, (2) Calcium Carbonate.
Answer:
(1) Sodium Oxide:
(2) Calcium Carbonate:
Question: Write the cations and anions present, if any in the following: CH3COONa, Nace, H2, NH4 NO3 .
Answer:
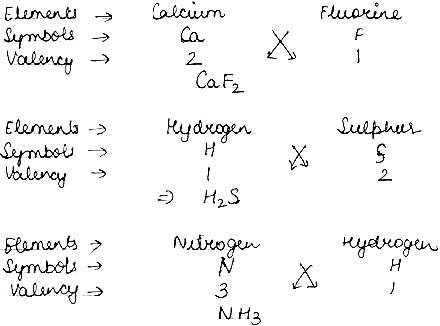
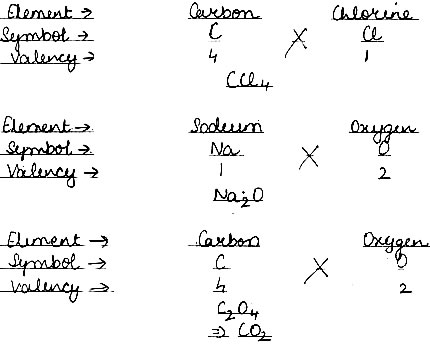
Question: Give the formula of the compound formed from the following sets of elements.
Answer:
Question: (I) What is an ion? How is it formed Explain with example of different ions.
(II) The valences (or charges) of some of the ions are given below:
IonValency
Sodium Ion1 =
Ammonium Ion1 +
Calcium Ion2 +
Lead Ion2 +
Bromide Ion1 –
Hydroxide Ion1 –
Sulphate Ion2 –
Phosphate Ion3 –
Using this information, write down the formula of the following compounds.
(1) Sodium Phosphate
(2) Ammonium Sulphate
(3) Calcium Hydroxide
(4) Lead Bromide
Answer: (I) An ion is a positively or negatively charged atom (or group of atoms). An ions is formed by the loss organ of electrons by an atoms. Ion with excess of electrons are anions and ions with less electron are cations.
Anions → Cl–, Br–
Cations → Na+, Mg2+
(II)
(1) Sodium Phosphate:
(2) Ammonium Sulphate:
(3) Calcium Hydroxide:
(4) Lead Bromide:
Question: Explain the formation of (1) Sodium ion,
(2) Chloride ion from their representative atoms given the no. of protons, electrons in each of them. What is the reason of +ve change on a sodium ion and a -ve change on a Chloride ion.
Answer: (1). A sodium atom loses 1 electron to form a sodium ion Na+, having 1 unit positive change
(2) A chloride atoms accepts 1 electron to form a chloride ion, Cl–, having 1 unit negative charge.
Na (-1 electron) Na+
Sodium atom ————→ Sodium ion
Protons = 11(+) Protons = 11(+)
Electrons = 11 (-) Electrons = 10 (-)
Overall Charge = 1+
Cl (+1 electron) Cl-
Chlorine atom ————→ Chlorine ion
Protons = 17(+) Protons = 17(+)
Electrons = 17 (-) Electrons = 18 (-)
Overall Charge = 1-
It is not possible for a sodium atom to gain 7 electrons as it requires a lot of energy so, it loses 1 to complete its shells.
It is not possible for a chlorine atom to lose 7 electrons So, it accepts 1 electron to get a negative change.
Question: (1) Write the symbols 1 formula of two simple ion and two compound ions (or polyatomic ions).
(2) An element Y has a valency of 4. Write the formula for its.
i) Chloride
ii) Oxide
iii) Sulphate
iv) Carbonate
v) Nitrate of the element
Answer:
(1). Simple ions → Cl–, Na+
Compound → CO32-, SO42-
(2)
i) Chloride:
Question: Calculation the formula masses of the following compounds:
(1) Calcium chloride
(2) Sodium carbonate
(Given: Atomic masses- Ca = 40 u, Cl = 35.5 u, Na = 23 u, C = 12 u, O = 16 u)
Answer: (1) Calcium chloride (Cacl2)
= 40 × 1 + 35.5 × 2
= 40 + 71
= 111 u.
(2) Sodium carbonate (Na2CO3)
= 23 × 2 + 12 × 1 + 16 × 3
= 46 + 12 + 48
= 106 u
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students