Question: What do you by elements, compounds and mixtures. Give Examples.
Elements:
- Consist of only one type of atom – which may, or may not join together to form molecules or large structures, therefore:
- Can exist as either atoms (e.g. argon) or molecules (e.g., nitrogen)
- Cannot be broken down into a simpler type of matter by either physical or chemical techniques – though some larger elements break-down spontaneously due to being radioactive.
Mixtures:
- consist of two or more different elements and / or compounds – physically intermingled,
- can be separated into their constituent parts by physical means (e.g. distillation of liquids or separating magnetic and non-magnetic solids using a magnet), and
- Have many of the properties of their constituent parts (e.g. the element “oxygen” is part of the mixture “air” and some of the properties of air are due to the oxygen, albeit somewhat reduced compared with pure oxygen due to the presence of the other constituents of the mixture called “air”).
Compounds:
- Consist of atoms of two or more different elements bound together chemically,
- Can be broken down into a simpler type of matter (elements) by chemical means; but not by physical means.
- Always contains the same ratio of component atoms.
- Have properties different from their component elements (e.g. the compound water (H2O) is a liquid at room temperature and pressure and has different chemical properties from those of the two elements, hydrogen (H2) and oxygen (O2), from which it is formed).
Question: What is mixture? Give examples of mixture.
Answer: Mixtures: A substance which contains two or more kind of particles is called a mixture. Eg: air and sugar solution.
There are many different types of mixtures, some of which have special names. These include:
- Homogeneous Mixtures: in which the two or more substances that form the mixture are evenly distributed throughout the mixture, e.g. vinegar is a homogeneous mixture of ethanoic acid and water.
- Heterogeneous Mixtures: in which the two or more substances that form the mixture are not evenly distributed throughout the mixture, e.g. oil and water.
- Solutions: a special type of homogeneous mixtures in which one substance (called the “solute”) is dissolved in another substance (called the “solvent”), e.g. salt water is salt dissolved in water – in such a way that the salt no-longer exists as solid particles within the water.
- Suspensions: heterogeneous fluid mixtures containing solid particles large enough for sedimentation, which means that the particles (compare with the “solute” part of a solution) will eventually settle to the bottom of the container (unlike in the case of colloids, below), e.g. particles of sand in water.
- Colloids: heterogeneous mixtures in which one is substance microscopically dispersed evenly throughout another substance (for comparison, the size of the particles of “solute” are greater than in the case of a solution, but much smaller than in the case of a suspension). There are many naturally occurring colloids, e.g. milk. Colloids are very important in biology and medicine.
- Alloys: mixtures in which the main element (or elements) are metal(s). A more technical definition of an alloy is “a partial or complete solid solution of one or more elements in a metallic matrix”. Common examples of alloys include bronze, brass and steels.
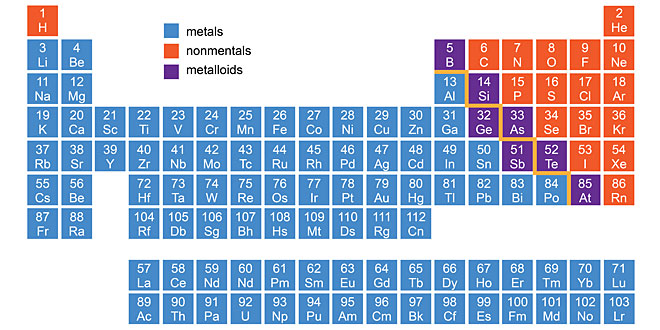
Question: Explain metals, metalloids and non non metals with two example each.
Answer:
Question: Out of a colloid, solution and a suspension:
1. Which one has the smallest particles?
Answer: Solution
2. Largest particles.
Answer: Suspension
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students