Question: How will you separate a mixture of oil and water?
Answer: To separate a mixture of oil and water, we need a separating funnel as both are immiscible liquids. Pour the mixture in separating funnel and let the funnel stand undisturbed for sometime. So that separate layer of oil and water are formed. Open the stopcock of the separating funnel and pour out the lower layer of water carefully.
Question: A student is given a mixture of naphthalene ball’s powder and common salt. He need to separate this mixture. How will he do this?
Answer:The properties of both naphthalene and common salt should be known, before we choose the separation technique.
Naphthalene is a sublimate which on heating changes to gaseous state directly. Hence to separate a volatile compound (sublimate) from a non-volatile compound (non-sublimate), the sublimation process is used.
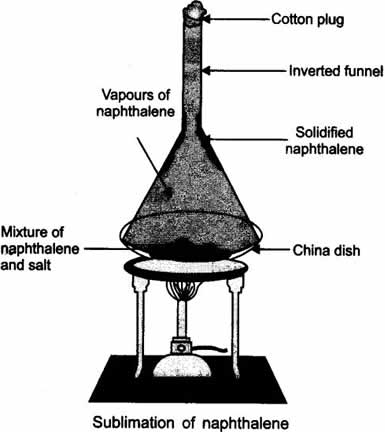
In a China dish the mixture is kept, and is placed on a stand. An inverted funnel is kept over the mixture in China dish with plugged stem. The sublimate on heating gets collected on the funnel and common salt remains in the China dish.
Question: How can we obtain different gases from air?
Answer: Air is a homogeneous mixture and its components can be separated by fractional distillation.
The flow diagram shows the steps involved in the process.
Air
↓
Compressed and cooled by increasing pressure and decreasing temperature.
↓
Liquid Air
↓
Allow to warm up slowly in fractional distillation column.
↓
Gases get separated at different heights.
↓
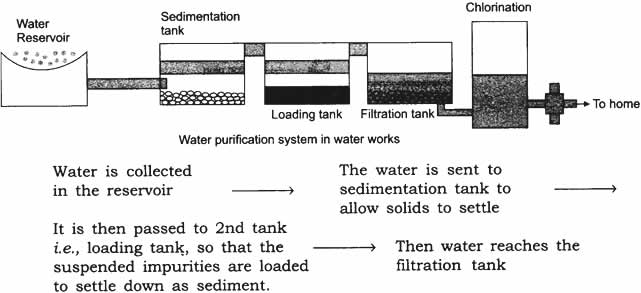
Question: Draw a flow diagram to show the water purification system in water works.
Answer:
In filtration tank water passes through different layers of sand and gravel as shown in the above figure this is for adsorption of impurities.
The clear water reaches a chlorinated tank where water is mixed with bleaching powder/chlorine to kill bacteria and then supplied to houses.
Question: Why is air considered as a mixture and not compound?
Answer: Air is considered as a mixture because it exhibits following properties:
- Each component present in air retains its properties.
- Each component can be separated by simple physical processes.
- The components do not have any fixed proportion. All gases are present in different amount. Example, in greener area:more oxygen and water vapour is present; near industrial area:air consists of lot of impurities and smoke suspended in it.
Question: How can you prove that water is a compound?
Answer: Water is a compound because if we pass electricity through it then at two different electrodes, we get two different gases i.e., oxygen and hydrogen during electrolysis of water. The ratio of oxygen: hydrogen is 1 : 2 by number of molecules.
- The properties of oxygen and hydrogen gases sire entirely different from that of liquid water.
- The ratio of oxygen: hydrogen combination is always constant i.e., 1: 2 by volume.
- To separate the components of water, we need electrolytic cell, and it is not a simple process.
Question: Give the difference between true solution, colloidal solution and suspension.
Answer: The difference between true solution, colloidal solution and suspension there is no more scope of solute particles to dissolve / dissociate into water. It is because the solute particle has taken all the inter molecular space present in the solvent.
On heating, the molecules of solvent gain kinetic energy, start vibrating and try to move away from each other thereby accommodating some more solute particle in this space and hence it becomes an unsaturated solution.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students