Question: Which contains more heat: 1 kg. of Ice at 0°C or 1 kg. of water of o°C? Give reason.
Answer: 1 kg. of water of contains more heat because it has latent heat stored in it.
Question: Which contains more heat, 1 kg. of water at 100°C or 1 kg. of steam of o°C? Give reason.
Answer: 1 kg. of steam contains more heat because when it was being changed from water to steam it had absorbed latent heat thus, becoming more hot.
Question: Convert 573 K. to Celsius.
Answer: We know:
Celsius = kelvin – 273
= 573 – 273
= 300°C
Question: Convert 373°C to kelvin.
Answer: We know:
Kelvin = Celsius + 273
= 373 + 273
= 646 K
Question: Name the temperature at which:
- A liquid changes into a gas.
- A solid change into a liquid.
Answer:
- Boiling Point
- Melting Point
Question: What is the name of process in which:
1. A solid directly changes into gas.
Answer: Sublimation
2. A gas directly changes into solid:
Answer: Sublimation
Question: What is the chemical name of dry Ice?
Answer: Solid Carbon Dioxide.
Question: Explain Why ice at 0°C is more effective in cooling than water at the same temperature?
Answer: Ice at 0°C has more capacity of absorbing latent heat because it is in solid state has to change to liquid state to absorb heat where has cold water is already in liquid state.
Question: Would you cool a bucket of water more quickly by placing it or ice or by placing ice int it?
Answer: Placing ice in the bucket of water would be better method because when ice is kept in water, the water is in direct contact with the ice, the rate of absorption of heat from water increases, making the water, cooler quickly.
Question: Why solid Carbon Dioxide called dry ice?
Answer: Solid Carbon Dioxide is a white solid and it is an extremely cold substance. Due to these properties of solid Carbon Dioxide we call it dry ice. Also, since solid carbon dioxide directly changed into gas without producing a liquid like ordinary ice, it is called “dry” ice.
Question: Explain why, steam at 100° is batter for healing purposes than boiling water at 100°C?
Answer: Steam contains latent heat so it is better for heating purposes.
Question: Which produces more sever burns: water or steam. Why?
Answer: Steam contains more latent heat so, it produces more sever burns.
Question: What is the physical state of water:
1. At 0°C
Answer: Solid
2. At 25°C
Answer: Liquid
3. At 100°C
Answer: Liquid
4. At 250°C
Answer: Gas
Question: What is melting point of a substance? What is melting point of ice?
Answer: The temperature at which a solid substance meets and changes into a liquid at atmosphere pressure is called melting point of the substance. The melting point of ice is 0°C.
Question: What is ‘boiling point’ of a substance? What is the boiling point of water?
Answer: The temperature at which a liquid substance boils and changes into a gas at atmospheric pressure is called the boiling point of the liquid. The boiling point of water is 100°C.
Question: Explain why, naphthalene balls kept in stored clothes, disappear over a period of time?
Answer: Naphthalene balls undergo Sublimation. They from naphthalene vapors and seem to disappear.
Question: How does perspiration cool our body?
Answer: When our body temperature tends too rise too much, our sweat glands give out moisture (or sweat) on our skin. When this sweat evaporates, it takes the latent heat of vaporization from our body. This keeps our body cool.
Question: Why does a desert cooler cool better on a hot, dry day?
Answer: The nigher temperature on a hot day increases the rate of evaporation of water and the dryness of air (low humidity of air) also increases the rate of evaporation of water. And due to increased rate of evaporation, a desert room cooler better on a hot and dry day.
Question: Why does our palm feel cold we put some ace-tome ( or perfume) on it?
Answer: When acetone changes from liquid to vapor state, it requires latent heat. It takes the latent heat from our hand and over palm feels cold.
Question: Define the term ‘latent heat of fusion’.
Answer: The latent heat of fusion of a solid is quantity of heat in foils required to convert 1 kg. of solid (at its meeting point) to liquids, without any change in temperature. The latent heat of fusion of ice is 3.34 × 105 fouls per kilogram.
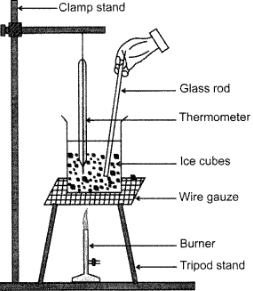
Question: Draw a well labelled diagram of experimental setup to study latent heat of fusion of ice.
Answer:
Question: Define the ‘latent heat of vaporization a liquid. What is the value of latent heat of vaporization of water?
Answer: The latent heat of vaporization of a liquid of a liquid is the quantity of heat in fouls required to convert 1 kg. of liquid to vapor or gas without change temperature. For water, it is 22.5 × 105 J/kg.
Question: What is sublimation? Name two substances which undergo sublimation.
Answer: The changing of a solid directly to its vapors on heating and of vapors into solid on cooling is known as sublimation.
Heating
Solid ———→ vapor (Gas).
←———
cooling
Substances like Camphor and Naphthalene undergo substance.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students



