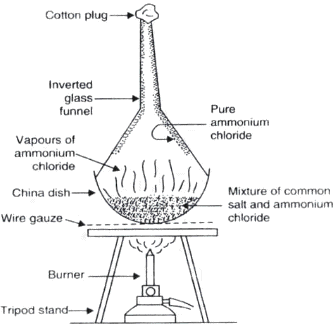
Question: Draw a labelled diagram of experimental setup to study latent heat demonstrate sublimation of ammonium chloride.
Answer:
Question: What are two ways in which physical states of water can be changed?
Answer: Physical states of matter can be changed into two ways:
- By changing temperature.
- By changing pressure.
- By increasing temperature solid can be changed to liquid and liquid to gas. By decreasing temperature gas can be changed to liquid and liquid to solid.
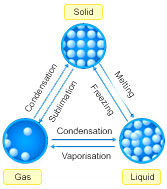
Question: Draw the states of matter triangle to show the interconnection of states of matter.
Answer:
Question: How can evaporation of liquid be made faster?
Answer: It can be made faster by:
- Temperature
- Increasing surface area
- Reducing humidity
- Increasing wind Speed
Question: What is evaporation? What are the factors which affect evaporation?
Answer: They are:
- Temperature
- Surface Area
- Humidity
- Wind speed
Question: Why does evaporation cool a liquid?
Answer: In a liquid, particles always have kinetic energy. Particles always have more kinetic energy. These particles absorb energy from liquid and evaporate and cool rest of the liquid.
Evaporation: The process of liquid changing into vapors even below its boiling point is called evaporation.
Question: Which of the following are matter? Chair, air, love, smell, hate, almonds, thought, cold, cold-drink, smell of perfume.
Answer: Chair, air, almonds and cold-drink.
Question: Give reasons for the following observation: The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food you have to go close.
Answer: The smell of hot sizzling food reaches severed metres away, as the particles of hot food have more kinetic energy and hence the rate of diffusion is more than the particles of cold food.
Question: A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Answer: A diver is able to cut through water in a swimming pool. This shows that the particles of water have intermolecular space and has less force of attraction.
Question: What are the characteristics of the particles of matter?
Answer: The characteristics of the particles of matter are:
- Particles have inter molecular space.
- Particles have inter molecular force.
- Particles of matter are moving continuously.
Question: The mass per unit volume of a substance is called density.
(density = mass/volume).
Arrange the following in order of increasing density: air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Answer: Increasing density: air < exhaust from chimneys < cotton < water < honey < chalk < iron.
Question: Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Answer: Comment on:
- Rigidity: The tendency of a substance to retain/maintain their shape when subjected to outside force.
- Impressibility: The matter has inter molecular space. The external force applied on the matter can bring these particles closer. This property is called impressibility. Gases and liquids are compressible.
- Fluidity: The tendency of particles to flow is called fluidity. Liquids and gases flow.
- Filling of a gas container: Gases have particles which vibrate randomly in all the directions. The gas can fill the container.
- Shape: Solids have maximum inter molecular force and definite shape.
Whereas liquids and gases takes the shape of container. - Kinetic energy: The energy possessed by particles due to their motion is called kinetic energy. Molecules of gases vibrate randomly as they have maximum kinetic energy.
- Density: It is defined as mass per unit volume, the solids have highest density.
Question: Give reasons
- A gas fills completely the vessel in which it is kept.
- A gas exerts pressure on the walls of the container.
- A wooden table should be called a solid.
- We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Answer:
- The molecules of gas have high kinetic energy due to which they keep moving in all directions and hence fill the vessel completely in which they are kept.
- A gas exerts pressure on the walls of the container because the molecules of the gas are in constant random motion due to high kinetic energy. These molecules constantly vibrate, move and hit the walls of the container thereby exerting pressure on it.
- The molecules / particles of wooden table are tightly packed with each other, there is no inter molecular space, it cannot be compressed, it cannot flow, all these characteristics are of solid. So wooden table should be called a solid. ‘We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert. It is because the molecules of air has less force of attraction between them and a very small external force can separate them and pass through it. But in case of solids, the molecules have maximum force of attraction, the particles are tightly bound due to this force. Hence large amount of external force is required to pass through solid.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students