Question: Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Answer: Ice is a solid but its density is lower than water due to its structure. The molecules in ice make a cage like structure with lot of vacant spaces, this makes ice float on water.
Question: Convert the following temperature to Celsius scale:
- 300 K
- 573 K
Answer:
- 1. 300 – 273 = 27°C
- 573 – 273 = 300°C
Question: What is the physical state of water at:
- 250°C
- 100°C
Answer:
- 250°C = gas
- 100°C liquid as well as gas
Question: For any substance, why does the temperature remain constant during the change of state?
Answer: During the change of state of any matter heat is supplied to the substance. The molecules of this matter use heat to overcome the force of attraction between the particles, at this period of time, temperature remains constant. This extra heat is acquired by the molecules in the form of hidden heat called latent heat to change from one state of matter to the other state.
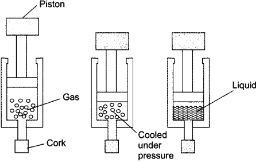
Question: Suggest a method to liquefy atmospheric gases?
Answer: The atmospheric gases are taken in a cylinder with piston fitted on it. By cooling and applying pressure on them, the gases can be liquefied.
Question: Why does a desert cooler cool better on a hot dry day?
Answer: The outer walls of the cooler get sprinkled by water constantly. This water evaporates due to hot dry weather. Evaporation causes cooling of inside air of cooler. This cool air is sent in the room by the fan.
Question: How does the water kept in an earthen pot (matka) become cool during summer?
Answer: The earthen pot is porous with lot of pores on it, the water oozes out through these pores and the water gets evaporated at the surface of the pot thereby causing cooling effect. This makes the pot cold and the water inside the pot cools by this process.
Question: Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Answer: Acetone, petrol or perfume evaporate when they come into contact with air. The evaporation causes cooling sensation in our hands.
Question: Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Answer: Tea in a saucer has larger surface area than in a cup. The rate of evaporation is faster with increased surface area. The cooling of tea in saucer takes place sooner than in a cup. Hence we are able to sip hot tea or milk faster from a saucer rather than a cup.
Question: What type of clothes should we wear in summer?
Answer: We should wear light colored cotton clothes in summer. Light colour because it reflects heat. Cotton clothes because it has pores in it, which absorbs sweat and allows the sweat to evaporate faster thereby giving cooling effect.
Question: Convert the following temperatures to the Celsius scale.
- 293 K
- 470 K
Answer:
- 293 K into °C 293 – 273 = 20°C
- 470 K into °C 470 – 273 = 197°C
Question: Convert the following temperatures to the Kelvin scale.
- 25°C
- 373°C
Answer:
- 25°C into K 25 + 273 = 298 K
- 373°C into K 4 373 + 273 = 646 K
Question: Give reason for the following observations.
- Naphthalene balls disappear with time without leaving any solid.
- We can get the smell of perfume sitting several meters away.
Answer:
- Naphthalene balls disappear with time without leaving any solid, because naphthalene balls sublime and directly changes into vapor state without leaving any solid.
- We can get the smell of perfume sitting several meters away because perfume contain volatile solvent and diffuse faster and can reach people sitting several meters away.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students