Question: If number of electrons in an atom is 8 and number of protons is also 8, then
(i) What is the atomic number of the atom? and
(ii) What is the charge on the atom?
Answer:
No. of electrons = 8
No. of protons = 8
(i) Atomic number = no. of protons = 8
(ii) As, Electrons = Protons
⊕ = Θ
∴ Atom is electrically neutral (No charge)
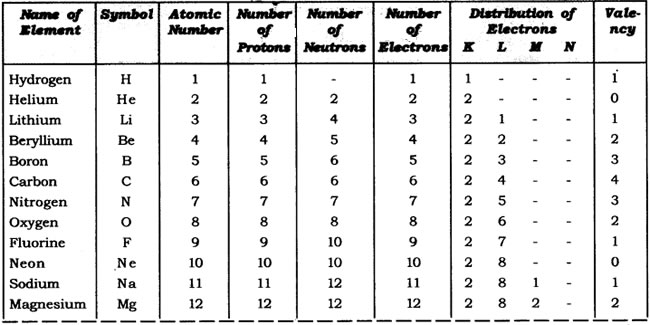
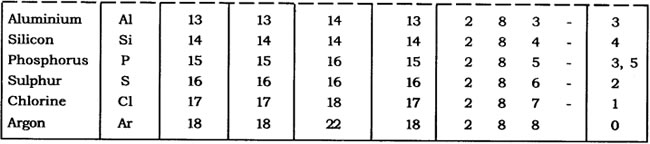
Question: With the help of given Table 4.1, find out the mass number of oxygen and sulphur atom.
Table: Composition of Atoms of the First Eighteen Elements with Electron Distribution in Various Shells
Answer:
Oxygen, No. of protons = 8
∴ No. of neutrons = 8
∴ Atomic number = 8
∴ Atomic mass number = P + N
= 8 + 8
= 16
Sulphur, No. of protons = 16
∴ No. of neutrons = 16
∴ Atomic number = 16
∴ Atomic mass number = P + N
= 16 + 16
= 32
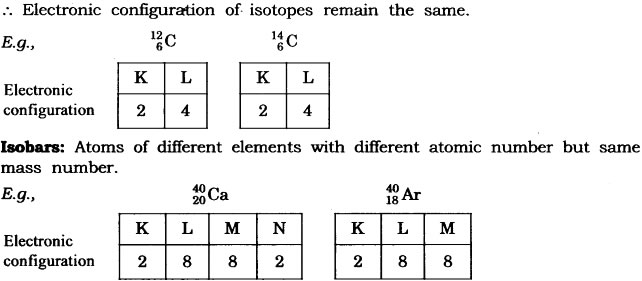
Question: Write the electronic configuration of any one pair of isotopes and isobar.
Answer: Isotopes: Atoms of same element having same atomic number but different mass number.
Question: Compare the properties of electrons, protons and neutrons.
Answer:
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students