Question: What are the limitations of J.J. Thomson’s model of the atom?
Answer: According to J.J. Thomson’s model of an atom, the electrons are embedded all over in the positively charged spheres. But experiments done by other scientists showed that protons are present only in the centre of the atom and electrons are distributed around it.
Question: What are the limitations of Rutherford’s model of the atom?
Answer: According to Rutherford’s model of an atom the electrons are revolving in a circular orbit around the nucleus. Any such particle that revolves would undergo acceleration and radiate energy. The revolving electron would lose its energy and finally fall into the nucleus, the atom would be highly unstable. But we know that atoms are quite stable.
Question: Describe Bohr’s model of the atom.
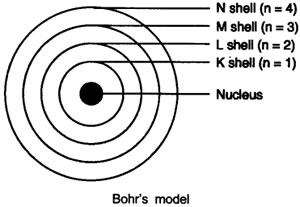
Answer: Bohr’s model of the atom
- Atom has nucleus in the centre.
- Electrons revolve around the nucleus.
- Certain special orbits known as discrete orbits of electrons, are allowed inside the atom.
- While revolving in discrete orbits the electrons do not radiate energy.
- These orbits or shells are called energy levels.
- These orbits or shells are represented by the letters K, L, M, N or the numbers n = 1, 2, 3, 4
Question: Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Answer: The rules for writing of distribution of electrons in various shells for the first eighteen elements are:
- The maximum number of electrons present in a shell is given by the formula – 2 n2
∵ n = orbit number i.e., 1, 2, 3
∵ Maximum number of electrons in different shells are:
K shell n = 1 2n2 => 2(1)2 = 2
L shell n = 2 2n2 => 2(2)2 = 8
M shell n = 3 2n2 => 2(3)2 = 18
N shell n = 4 2n2 => 2(4)2 = 32 - The maximum number of electrons that can be accommodated in the outermost orbit is 8.
- Electrons are not accommodated in a given shell unless the inner shells are filled. (Shells are filled step-wise).
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students