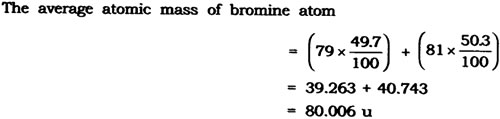Question: Define valency by taking examples of silicon and oxygen.
Answer: Valency is the combining capacity of an atom.
Atomic number of oxygen = 8 Atomic number of silicon = 14 K L M
Electronic configuration of oxygen = 2 6 –
Electronic configuration of silicon =2 8 4
In the atoms of oxygen the valence electrons are 6 (i.e., electrons in the outermost shell). To fill the orbit, 2 electrons are required. In the atom of silicon, the valence electrons are 4. To fill this orbit 4 electrons are required.
Hence, the combining capacity of oxygen is 2 and of silicon is 4.
i.e., Valency of oxygen = 2
Valency of silicon = 4
Question: Explain with examples: (i) Atomic number (ii) Mass number,(iii) Isotopes and (iv) Isobars. Give any two uses of isotopes.
Answer:
(i) Atomic number: The atomic number of an element is equal to the number of protons in the nucleus of its atom. e.g., Oxygen has 6 protons hence atomic no. = 6.
(ii) Mass number: The mass number of an atom is equal to the number of protons and neutrons in its nucleus.
Nucleons = number of protons + number of neutrons Example: Protons + Neutrons = Nucleus = Mass number 6 + 6 = 12
(iii) Isotopes: Isotopes are atoms of the same element which have different mass number but same atomic number.
(iv) Isobars: Isobars are atoms having the same mass number but different atomic numbers.
Both calcium and argon have same mass number but different atomic number.
Two uses of isotopes are:
- An isotope of iodine is used in the treatment of goitre.
- An isotope of uranium is used as a fuel in nuclear reactors.
Question: Na+ has completely filled K and L shells. Explain.
Answer: Sodium atom (Na), has atomic number =11
Number of protons =11
Number of electrons = 11
Electronic configuration of Na = K L M – 2 8 1
Sodium atom (Na) looses 1 electron to become stable and form Na+ ion. Hence it has completely filled K and L shells.
Question: If bromine atom is available in the form of say, two isotopes 7935Br (49.7%) and 8135Br (50.3%), calculate the average atomic mass of bromine atom.
Answer:
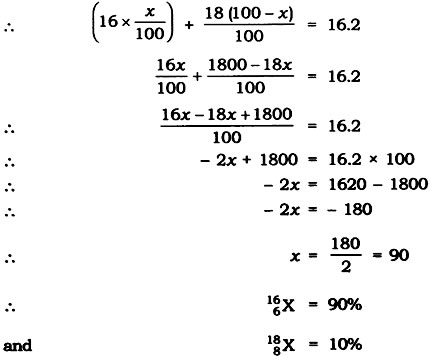
Question: The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 168X and 188X in the sample?
Answer: Let the percentage of 168X be x and the percentage of 168X be 100 – x.
Question: If Z = 3, what would be the valency of the element? Also, name the element.
Answer: Z = 3, (i.e, atomic number —> z)
∴ Electronic configuration = 2, 1
Valency = 1
Name of the element is lithium.
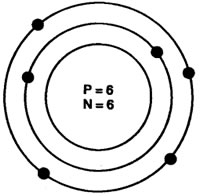
Question: Composition of the nuclei of two atomic species X and Y are given as under
X – Y
Protons =6 6
Neutrons = 6 8
Give the mass number of X and Y. What is the relation between the two species?
Answer: Mass number of X = Protons + Neutrons
= 6 + 6 = 12
Mass number of Y = Protons + Neutrons = 6 + 8 = 14
As the atomic number is same i.e., = 6.
[atomic number = number of protons].
Both X and Y are isotopes of same element.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students