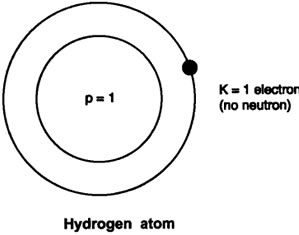
Question: Draw the atomic structure of hydrogen atom.
Answer:
Question: Why are some elements chemically inert?
Answer: Because their outermost shell is completely filled.
Question: Why is atom electrically neutral?
Answer: It has same number of protons and electrons, (positive charge = negative charge).
Question: What is the charge and mass of a-particles?
Answer: Charge is + 2
Mass is 4 a.m.u.
Question: What are valence electrons?
Answer: Electrons present in the outermost shell of an atom are called valence electrons.
Question: An atom has atomic number 12, what is its valency and name the element?
Answer: Atomic number = 12
∴ Protons = Electrons = 12 Electrons Configuration = K L M -2 8 2
∴ Valency = 2
Element is magnesium.
Question: Find the number of neutrons in 2713X.
Answer: Mass number = 27
∴ p + n = 27 p = 13, (Atomic No. = Number of protons)
∴ 13 + n = 27
∴ n = 14
∴ Neutron =14
Question: Where is the mass of atoiji concentrated?
Answer: Mass of an atom is concentrated in nucleus.
Question: Name two elements with same number of protons and neutrons?
Answer: Carbon (Protons = Neutrons = 6)
Oxygen (Protons = Neutrons = 8)
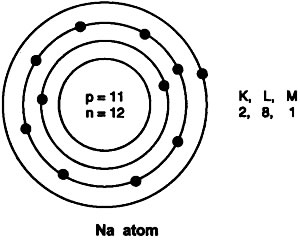
Question: Draw the atomic structure of sodium atom.
Answer:
Question: Name the isotope used for treatment of cancer.
Answer: Isotope of cobalt.
Question: AZX What does this symbol represent?
Answer: X —> Symbol of element
A —> Mass number
Z —> Atomic number
Question: Can the value of ‘Z’ be same for two different atoms?
Answer: No, (Z = atomic number), two different atoms cannot have same atomic number.
Question: Can the value of A’ be same for two different atom?
Answer: Yes, it can be e.g. Ca and Ar has A-40 (i.e., mass number).
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students