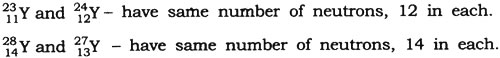Question: Name the scientist who discovered protons and neutrons in an atoms.
Answer: Protons were discovered by E. Goldstein in 1866 and neutrons were discovered by J, Chadwick in 1932.
Question: What is the contribution of Bohr and Bury together in the structure of atom’s explanation?
Answer: Both Bohr and Bury gave the distribution of electrons into different atoms by giving the formula 2n2, where n = shell number.
Question: Draw the atomic structure of (i) an atom with same number of sub-atomic particles, (ii) an atom with same number of electrons in L and M shell.
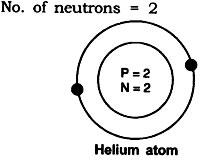
Answer: (i) An atom with same number of sub-atomic particles is 24He
No. of protons = 2
No. of electrons = 2
(ii) An atom with L and M shell filled —->K L M- 2 8 8
Question: What is an octate? Why would atoms want to complete their octate?
Answer: When the outermost shell of an atom i.e., L, M or N are completely filled with 8 electrons in the shell, it is said an octate. Atoms would want to complete their octate because they want to become stable.
Question: Find the valency of 147N and 3517Cl.
Answer: The atomic number of nitrogen = 7, No. of protons = 7, No. of electrons = 7
Electronic configuration = K L M =2 5 –
Valency = 3
Because either it will gain three electrons or share 3 electrons to complete its octate.
The atomic number of chlorine = 17, p = 17, e=17
Electronic configuration = K L M= 2 8 7
Valency = 1
Because it will gain 1 electron to complete its octate.
Question: Pick up the isotopes among the following and state reason.
![]()
Answer: The isotopes are 3517X and 3717X as both the atoms show same atomic number but different mass number.
Question: Pick up atoms which have same number of neutrons from the following:
Answer:
Question: What are nucleons? What is the name given to those atoms which have same number of nucleons in it?
Answer: Protons and neutrons present in the nucleus are called nucleons Isobaric elements have same number of nucleons in it.
Question: Give the difference between three sub-atomic particles.
Answer: Three sub-atomic particles are electron, proton and neutron
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students