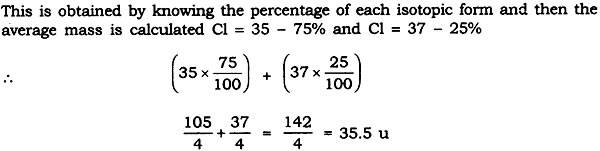Question: Give the names of three atomic species of hydrogen.
Answer: Three atomic species of hydrogen are:
Question: Atomic Mass exists as whole number, why do we write the atomic mass of chlorine as 35.5 u.
Answer: Chlorine has two isotopes and the mass of an atom is taken as the average mass of all the naturally occurring atoms of that element.
Question: Give difference between isotopes, and isobars.
Answer:
Isotopes:
- Are atoms of same element
- Have same atomic number
- Have different mass number
- Number of protons and electrons are same in these atoms.
Isobars:
- Are atoms of different element
- Have different atomic number
- Have same mass number
- Number of protons and electrons are not same in these atoms.
Question: Number of protons and electrons are same in an atom. Then why is it wrong to say that atomic number of an atom is equal to its number of electrons.
Answer: Atomic number ≠ Number of electrons, although number of protons = number of electrons because the electron’s number can change in an atom by loss, or gain of it. But the proton’s number remain constant (as it does not take part in loss or gain).
Question: An atom is electrically neutral, on loss or gain of electrons why does it become charged?
Answer: An atom is electrically neutral because of same number of protons and electrons. But it becomes charged, to become stable atom, loses or gains electrons. Hence,
Number of protons ≠ Number of electrons
If it loses electrons p > e; hence +ve charge is obtained.
If it gains electrons e > p; hence -ve charge is obtained.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students