Question: With the help of an activity in daily life, how can you prove that atoms are divisible.
Answer: Activity
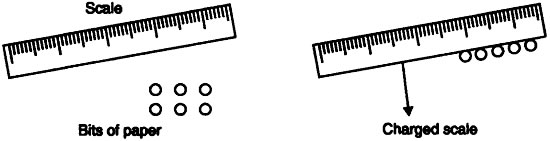
- Take a scale, rub it on hair, try to attract a small bit of paper.
- Now divide the bit of paper further into smaller pieces.
- Again bring the charged scale near to this pieces of papers.
- You will observe that the bits of paper still get attracted.
Conclusion: This activity shows that atom contains charges and these charges are opposite in nature which shows the attraction. Hence here scale and paper both are oppositely charged and hence attract each other. Also, every atom has at least one sub-atomic particle.
Question: In the structure of an atom why are protons present in the centre and are not pulled outside by the electrons as both are oppositely charged with same unit of charge?
Answer: Protons are heavy with mass 1 unit and hence are concentrated in the centre
of the atom. The mass of electrons is negligible i.e.1/1800 times less than that of protons. Hence are not able to attract the protons and pull them out of the nucleus, although their charge is of same value.
Question: According to you, among the structure of atom studied which model is correct and why?
Answer: Bohr’s model of an atom is the best model and is correct because it gives the explanation of nucleons (protons and neutrons) in the centre and how electrons revolve around the nucleons in their discrete, special orbits, so electrons don’t loose/radiate energy and remain bonded in their shell.
Question: Give an activity to understand the implications of Rutherford’s a scattering experiment by a gold foil.
Answer: To understand the implications of Rutherford’s a-particle scattering experiment:
Activity: Let a child stand in front of a wall with his eyes closed. Let him throw stones at the wall from a distance. He will hear sound for each strike of stone on the wall. This is like a nucleus of the atom. But if a blind-folded child has to throw stones at a barbed-wire fence, most of the stones would not hit the fencing and no sound would be heard.
This is because there are lots of gap in the fence which allows the stone to pass through them. This is like empty space in an atom through which a-particles will pass through. Based on the above activity and similar reasoning Rutherford concluded the a-particle scattering experiment as:
- Most of the space inside the atom is empty as a-particles passed through the foil.
- Very few particles deflected from their path, this show that positive charge occupies less space.
- A very small fraction of a-particles are deflected by 180°, this shows that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
Question: What are isotopes? State its characteristics, give uses of isotopes?
Answer: Atoms of same element with same atomic number but different mass number are isotopes.
Characteristics:
- Physical properties of the isotopes are different e.g. mass, density.
- Chemical properties of the isotopes are same due to same number of electrons.
Uses:
- Uranium isotope is used as a fuel in nuclear reactor (U-235).
- Cobalt isotope is used for treatment of cancer (Co-60).
- Iodine isotope is used in the treatment of goitre.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students