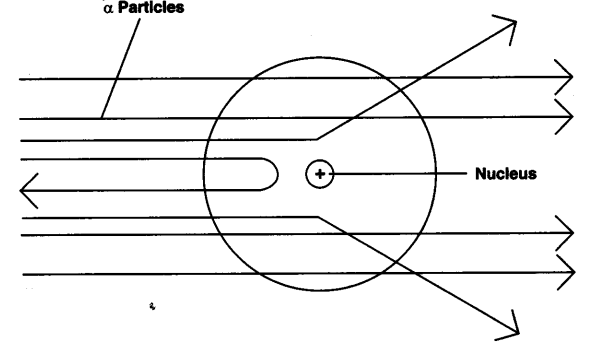
Question: Explain Rutherford’s α-particle scattering experiment and give its observation and conclusion drawn.
Answer: Rutherford’s α-particle scattering experiment:
Fast moving α-particles were made to fall on a thin gold foil. Particles have + 2 charge and 4u mass, and considerable amount of energy.
Observations:
- Most of the α-particles passed straight through the foil.
- Some of the α-particles were deflected by small angles by the foil.
- One out of every 12000 particles rebounded.
Conclusion from observation:
- Most of the space inside the foil is empty.
- Positive charge of atom occupies very less space.
- Mass of the atom is concentrated in the centre with all positive charge concentrated in small volume within the atom.
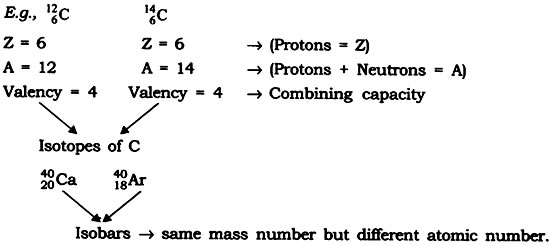
Question: Establish the relationship between atomic number, mass number, isotopes, isobars and valency of an atom.
Answer: Atomic number — Gives the number of protons (Z)
Mass number — Gives the number of protons and neutrons (A)
Isotopes — When atoms of same element have same number of protons (Z) but different number of a neutrons (s) such atoms are called isotopes.
Isobars — When atom of different element have same mass number (A) but different atomic number (Z) such atoms are called isobars.
Valency — It is the combining capacity of an atom.
Question: Give the evidence for existence of nucleus in an atom.
Answer: When Rutherford bombarded thin sheet of gold foil with α -particles (α-particles are doubly charged helium ions, He2+), he found that:
(i) Most of the α-particles passes through the foil without any deflection. He calculated that only one particle in 105 bounced back. This shows that most of the space inside the atom is empty and hollow.
(ii) Some of the α-particles were deflected through various angles while a very small number were actually deflected by as much as 180°.
This shows that:
- There is a heavy positively charged centre inside the atom. This centre is known as nucleus.
- Since only a very small fraction of α-particles were deflected through large angles, the nucleus is situated in a very small volume of the atom and is positively charged. The nucleus was found to be about 105 times smaller than the total area occupied by the atom as a whole.
- Since α-particles deflected by the nucleus have an appreciable mass, it means that the entire mass of the atom lies inside the nucleus.
Question: How was it shown that an atom has a lot of empty space within it?
Answer: On passing alpha particles through a thin gold foils, the alpha particles pass straight through the gold atoms of gold foil. This shows that an atom has a lot of empty space within it.
Question: What is a proton? State its relative mass and charge.
Answer: The positively charged particle found in the atoms of all elements in known as proton. The relative mass of a proton is 1 u and charge is + 1 (plus one).
Question: All the gases from cathode rays and anode when electrically is passed through them:
(1). What does the formation of cathode rays tell us about atoms?
(2). What does the formation of anode rays tell us about atoms?
Answer:
- The formation of cathode rays tell us about the presence of electrons in atoms of elements.
- The formation of anode rays tell us about the existence of protons in atoms of elements.
Question: What do you understand by the term electronic configuration of an element? Write down the electron configuration of oxygen. (atomic no. of oxygen = 8).
Answer: The arrangement off electron in the various shells (or energy levels) of an atom of the element is known as electron configuration.
Atomic number of Oxygen = 8
No. of protons = No. of electrons = 8
So, electronic configuration = K,L
= 2, 6.
Question: An element has an atomic number 12. How many electrons will be present in K, L & M energy shells?
Answer: Atomic number = 12
Number of protons = 12
No. of electrons = 12
So, electronic configuration = K, L, M
= 2, 8, 2
Question: An element has an atomic number 13 and an atomic mass of 27.
(1). How many electrons are their in the stone.
(2). How are these electrons distributed in the various energy shells.
Answer: (1). Atomic Number = No. of P+ = 13
Atomic mass = 27
Electrons = No. of P+ = 13
So, it has 13 electrons
(2). So, electronic configuration = K, L, M
= 2, 8, 3
Question: (1). What are cathode rays? What is the nature of charge on cathode rays?
(2). Explain how cathode rays formed from the gas taken in the discharge tube.
(3). What conclusion is from cathode rays?
Answer:
- On passing electricity of high voltage through a gas at very law pressure, streams of minute small, negatively charged particles called electrons are given out by the cathode. These streams of particles are called cathode rays. Cathode rays have a negative charge on them.
- The gas taken in the discharge tube contain electrons. When high electrical voltage is applied, the electrical energy pushes out some of the electrons from the atoms of the gas these fast moving electrons for cathode rays.
- All gases have small, negatively cWhiharged subatomic particles called electrons.
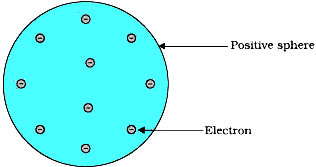
Question: (I). Describe Thomson’s model of the atom. Which subatomic particles were not present in his modal of atoms.
(II). The mass number of an element is 18. It contains 7 electrons. Give no. of p+ and n. What is the atomic number of it?
Answer: (I).
The Thomson’s model of an atom tell us:
- An atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it.
- The positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.
(II). No. neutrons = Mass Number – no of electron
= 18 – 7
= 11
No of protons = No. of electrons = 7.
Question: (I). Describe the Rutherford’s model of an atom state one draw back of it.
(II). Mass Number = 23; 11 electrons. Find neutrons and protons. Atomic No = ?
Answer: (I). According to Rutherford’s model of the atom:
- An atom consists of a positively charged dense & small nucleus containing protons and nucleus. Almost entire mass of atoms is concentrated in nucleus.
- Nucleus is surrounded by negatively charged electrons. They revolve in circular paths at very high speeds. These paths are called orbits.
- The electrostatic attraction between positively charged nucleus & negatively charged electrons holds the atoms together.
- Due to equal number of electrically.
- Most of the atom is empty space.
Rutherford could not explain stability of atom.
(II) Mass Number = 23
Electrons = 11
Neutrons = ?
Protons = ?
Atomic Number = ?
Electrons = Protons = Atomic Number = 11
Neutrons = Mass Number – Protons
= 23 – 11
= 12
Ans. = 12; 11; 11
Question: An element has an atomic number 11 and its mass number is 23. What is the arrangement of electrons in the shells? State the nuclear composition of an atom of the element.
Answer: Atomic Number = 11
Mass Number = 23
Neutrons = Mass Number – Atomic Number
= 23 – 11
= 12
Protons (atomic no.) = 11
Electron = Protons
= 11
Electronic configuration = K, L, M
= 2, 8, 1
Nuclear composition = Protons, Nuetrons
= 11, 12
Question: (1). What is the relation between atomic number and mass number of an element?
(2). If an element M has mass number 24 and atomic no 12, how many are neutrons?
Answer: (1). Mass Number = No. of protons + No. of neutrons (1).
But No. of protons = Atomic Number (2).
∴ Mass Number = Atomic Number + No of neutrons. [From (1) & (2)]
(2). Mass Number = 24
Atomic No. = 12.
Now,
Mass Number = Atomic Number + No of neutrons.
24 = 12 + n
24 – 12 = n
12 = n
So, number of neutrons = 12
Question: The nucleus of an atoms has 5 protons and 6 neutrons. What would be the (1) atomic number, (2) Mass number, (3) The number of electrons and, (4) The number of valence electrons, per atom of this element?
Answer:
- Atomic number = no. of protons
= 5 - Mass number = no. of protons + neutrons
= 5 + 6
= 11 - Electrons = no. of protons
= 5 - Valence electrons = K, L
2, 3
= 3
Question: What valencies wile be shown by the elements A, B, C, D and E having atomic numbers 2, 4, 8, 10 and 13 respectively.
Answer:
- A
Atomic Number = 2
No. of electrons = 2
Valence electrons = 2 (K shell)
valency = 0 - B
Atomic Number = 4
Valence electrons = 2 (L shell)
valency = 2 (giving away). - C
Atomic Number = 8
Valence electrons = 6 (L shell)
valency = 2 (Taking). - D
Atomic Number = 10
Valence electrons = 8 (L shell)
valency = 0 - E
Atomic Number = 13
Valence electrons = 3 (M shell)
valency = 3
Question: What is the reason for different atomic masses of isotopes.
Answer: Atomic mass consists of mass of neutron & protons. The number of neutrons differ in isotopes. So they have different masses of isotopes.
Question: What is the reason for the slightly different properties of all isotopes of elements?
Answer: There is a difference of physical properties between isotopes, because physical properties depend upon mass and mass of isotopes differ due to the unequal number of neutrons.
Question: Hydrogen has three isotopes.

Explain why: (1). These isotopes have identical chemical properties.
(2). They are electrically neutral.
Answer:
- All isotopes of Hydrogen have same electronic configuration so, these isotopes have identical chemical properties.
- This is because the change is only in the number of neutrons which have no charge. The number of electrons & protons remains the same.
Question: Given that the percentage of the abundance of isotope 20/10 Ne is 90% and that of the isotope 22/10 Ne is 10%. Calculate average atomic Mass.
Answer: Average atomic mass =
⇒ 20 × 90/100 + 22 × 10/100
⇒ (18 + 2.2) u
⇒ 20.2 u
Question: For the symbols H, D ant T write the subatomic particles (protons, neutrons and electrons) found in each one of them.
Answer:
Mass number = 1
No. of protons = 1
Neutrons = 0
Electrons = 1
(2). D or
Mass number = 2
No. of protons = 1
Neutrons = 1
Electrons = 1
(3). T or
Mass number = 3
No. of protons = 2
Neutrons = 1
Electrons = 1
Question: An element has Z = 7. What is the valency of the element? Also name the element.
Answer: (Z) Atomic Number = 7
No. of protons = 7
No. of electrons = 7
Electronic Configuration = K, L
2, 5
Valency = 3
So, element is Nitrogen.
Question: Give the number of protons, neutrons and electrons per atom in the two isotopes of chlorine 35/17 Cl and 37/17 Cl.
Answer: (1). 35/17 Cl.
Mass number = 35
Atomic Number = 17
protons = 17
Neutrons = 35 – 17
= 18
Electrons = 17
(2).37/17 Cl.
Mass number = 37
Atomic Number = 17
protons = 17
Neutrons = 37 – 17
= 20
Electrons = 17
Question: (1). What are radioactive isotopes ? Give two examples.
(2). Give any two uses of it.
(3). An element Z contains 2 naturally occurring isotopes 35/17 Z and 37/17 Z. If the average atomic mass of this element be 35.5 u, calculate the percentage of two isotopes.
Answer: (1). The isotopes which are unstable (due to the presence of extra neutrons in their nuclei) and emit various types of radiations are called radioactive isotopes (or first radioisotopes).
(2). Uses of isotopes are:
- Radioactive isotopes (Cobalt – 60) are used in the treatment of cancer.
- Radioactive isotopes are used to determine the activity of thyroid gland which helps in the treatment of diseases like goitre. (Iodine – 131)
(3). Let percent of 35/17 Z be x
So percentage of 37/17 Z be 100 – x
S0,
35 × x/100 + 37 × 100 – x)/100 = 355/10
7 × x/20 + 3700 – 37 x/100 = 355/10
7 x/20 + 3700 – 37 x/100 = 355/10
35 x + 3700 – 37 x/100
35 x – 37 x = 3550 – 3700
– 2 x = – 150
x = 75%
So percentage of 35/17 Z = 75%
37/17 Z = 100 – 75 = 25%
Question: (1). Define valency of an element. What valency will be shown by an element having atomic number 4.
(2). What is the relation between the valency of an element and No. of valence electrons in its atoms? Explain with Eg.
Answer: (1). Valency of an element is defined as its combining capacity.
Atomic no. = 14
protons = 14
Electrons = 14
Electronic Configuration = K, L, M
2, 8, 4
∴ its valency is 4.
(2). The valency of an element is decided by the “number of valence electrons” in its atom. The valency of an element is either equal to the no. of valence electrons in its atom or equal to the no. of electrons required to complete 8 electrons is the valence shell.
Eg: Sodium has 1 valence electron & the valency of sodium is also 1. So, in the case of Sodium the valency is equal to the no. of valence electrons in its atom.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students