10 Class Science Board Exam 2025:
Note:
- Please check that this question paper contains 23 printed pages.
- Please check that this question paper contains 39 questions.
- Q.P. Code given on the right hand side of the question paper should be written on the title page of the answer-book by the candidate.
- Please write down the Serial Number of the question in the answer-book before attempting it.
- 15 minute time has been allotted to read this question paper. The question paper will be distributed at 10.15 am. From 10.15 a.m. to 10.30 a.m., the students will read the question paper only and will not write any answer on the answer- book during this period.
| School Name: | New Delhi 110085 India |
| Class: | 10th Standard (CBSE) |
| Subject: | Science |
| Time Duration: | 03 Hours |
| Maximum Marks: | 80 |
| Date: | 20 February, 2025 |
General Instructions:
Read the following instructions very carefully and strictly follow them:
- This question paper comprises 39 questions. All questions are compulsory,
- This question paper is divided into five sections – A, B, C, D and E.
- Section A – Question Nos. 1 to 20 are multiple choice type questions. Each question carries 1 mark.
- Section B – Question Nos. 21 to 26 are very short answer type questions. Each question carries 2 marks. Answers to these questions should be in the range of 30 to 50 words.
- Section C – Question Nos. 27 to 33 are short answer type questions. Each question carries 3 marks. Answers to these questions should be in the range of 50 to 80 words.
- Section D – Question Nos. 34 to 36 are long answer type questions. Each question carries 5 marks. Answers to these questions should be in the range of 80 to 120 words.
- Section E – Question Nos. 37 to 39 are of 3 source-based / case-based units of assessment carrying 4 marks each with sub-parts.
- There is no overall choice. However, an internal choice has been provided in some sections. Only one of the alternatives has to be attempted in such questions.
Section – A
Select and write the most appropriate option out of the four options given for each of the questions 1 to 20. There is no negative marking for wrong answers. Each question carries 1 mark.
Question: 01. In which one of the following situations a chemical reaction does not occur?
- Milk is left open at room temperature during summer
- Grapes get fermented
- An iron nail is left exposed to humid atmosphere
- Melting of glaciers
Question: 02. In order to prepare dry hydrogen chloride gas in humid atmosphere the gas produced is passed through a guard tube (drying tube) which contains:
- Calcium chloride
- Calcium oxide
- Calcium hydroxide
- Calcium carbonate
Question: 03. The property by virtue of which a solid material can be drawn into thin wires is called:
- malleability
- ductility
- rigidity
- resistivity
Question: 04. Select from the following a hydrocarbon having one C-C bond and one C=C bond:
- Benzene
- Cyclohexane
- Butynes
- Propyne
Question: 05. The essential element taken up from the soil by the plants to synthesize proteins is:
- Phosphorus
- Nitrogen
- Iron
- Magnesium
Question: 06. Select TRUE statements about lymph from the following:
A. Lymph vessels carry lymph through the body and finally open into larger arteries.
B. Lymph contains some amount of plasma, proteins and blood cells.
C. Lymph contains some amount of plasma, proteins and red blood cells.
D. Lymph vessels carry lymph through the body and finally open into larger veins.
The true statements are:
- A and B
- B and D
- A and C
- C and D
Question: 07. Plants like rose and banana have lost the capacity to produce:
- (a) flowers
- (b) buds
- (c) seeds
- (d) fruits
Question: 08. In a bisexual flower the male gametes are present in the :
- (a) anther
- (b) ovary
- (c) stigma
- (d) filament
Question: 09. When a pure-tall pea plant is crossed with a pure-dwarf pea plant, the percentage of tall pea plants in and generation pea plants will be respectively :
- (a) 100% ; 25%
- (b) 100% ; 50%
- (c) 100% ; 75%
- (d) 100%; 100%
Question: 10. To get an image of magnification on a screen using a lens of focal length 20 cm, the object distance must be:
- (a) Less than 20 cm
- (b) 30cm
- (c) 40 cm
- (d) 80cm
Question: 11. An optical device ‘X’ is placed obliquely in the path of a narrow parallel beam of light. If the emergent beam gets displaced laterally, the device ‘X’ is:
- (a) plane mirror
- (b) convex lens
- (c) glass slab
- (d) glass prism
Question: 12. A piece of wire of resistance ‘R’ is cut lengthwise into three identical parts. These parts are then connected in parallel. If the equivalent resistance of this combination is R’, then the value of R/R’ is :
- (a) 1/9
- (b) 1/3
- (c) 3
- (d) 9
Question: 13. An electric bulb is rated 220 V; 11 W. The resistance of its filament when it glows with a power supply of 220 V 1s: .
- (a) 4400 ohm
- (b) 440 ohm
- (c) 400 ohm
- (d) 20 ohm
Question: 14. The minimum number of identical bulbs of rating 4V, 6W, that can work safely with desired brightness, when connected in series with a 240 V mains supply is:
- (a) 20
- (b) 40
- (c) 60
- (d) 80
Question: 15. In the food chains given below. Select the most efficient food chain in terms of energy:
- (a) Grass > Grasshopper Frog > Snake
- (b) Plants > Deer > Lion
- (c) Plants > Man
- (d) Phytoplankton > Zooplankton > Small Fish > Big Fish
Question: 16. Which one of the following gets biomagnified at different levels in a food chain?
- (a) Carbon monoxide
- (b) CFC’s
- (c) DDT
- (d) Manure
Question Nos. 17 to 20 consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option (a), (b), (c) and (d) as given below:
- Both, Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of Assertion (A).
- Both, Assertion (A) and Reason (R) are true, and Reason (R) is not the correct explanation of Assertion (A).
- Assertion (A) is true, but Reason (R) is false.
- Assertion (A) is false, but Reason (R) is true.
Question: 17.
Assertion (A): In large animals, oxygen can reach different parts of the animal’s body easily.
Reason (R): Respiratory pigments take up oxygen from the air and carry it to body tissues. 1
Question: 18.
Assertion (A): Concentrated nitric acid is diluted by adding water slowly to acid with constant stirring.
Reason (R): Concentrated nitric acid is easily soluble in water.
Question: 19.
Assertion (A): In reptiles, the temperature at which the fertilized eggs are kept decides the sex of the offspring.
Reason (R): Sex is not genetically determined in some animals. 1
Question: 20.
Assertion (A): When ciliary muscles contract, the eye lens becomes thin.
Reason (R): Ciliary muscles control the power of the eye lens. 1
Section – B
Question Nos. 21 to 26 are very short answer type questions. Each question carries 2 marks.
Question: 21. Define oxidation. Identify and name the substance oxidised in the following reaction : CuO + H2 H20
Question: 22.
(A) Show the formation of magnesium chloride by electron transfer. Write the name of the cation and anion present in the compound formed. (Atomic Number of Mg — 12. CI 17)
OR
(B) I Iow is zinc extracted from its ore? Name the processes involved in the extraction and write chemical equations for the reactions that occur during these processes.
Question: 23. “Plants use a variety of techniques to get rid of waste material.” Justify this statement giving any four ways.
Question: 24. Explain with the help of a flow chart that in human beings father is responsible for the sex (male or female) of the child.
Question: 25.
(A) Draw a ray diagram to show the refraction of a ray of light passing through an equilateral glass prism. Mark the angle through which the emergent ray bends from the direction of the incident ray and also name it.
OR
(B) Name the type of lenses required by the persons for the correction of their defect of vision called presbyopia. Write the structure of the lenses commonly used for the correction of this defect giving reason for such designs.
Question: 26. What are magnetic field lines. List two important properties of magnetic field lines.
Section – C
Question Nos. 27 to 33 are short answer type questions. Each question carries 3 marks.
Question: 27. (A) Why do we balance a chemical equation? Name and state the law that suggests the balancing of a chemical equation? Balance the following chemical equation:
Zn + H3PO4 > Zn3 (PO4)2 + H2
OR
(B) Define a precipitation reaction. Give its example and also express the reaction that occurs in the form of a balanced chemical equation.
Question: 28. Design an activity to show that metals are good conductors of heat and have high melting points.
Question: 29. The digestion of food in the human alimentary canal is a complex process. State the enzyme/salt present in the following and mention their function in the process of digestion.
(i) Saliva
(ii) Bile Juice
(iii) Pancreatic Juice
Question: 30. State two limitations? of electrical impulses in multicellular organisms. Why is chemical communication better than electrical impulses as a means of communication between cells in multicellular organisms?
Question: 31. If we want to obtain a virtual and magnified image of an object by using a concave mirror of focal length 18 cm, where should the object be placed? Use mirror formula to determine the object distance for an image of magnification +2 produced by this mirror to justify your answer.
Question: 32. The electrical resistivity of three materials A, B and C at 20°C is given below:
- Classify these materials as conductor, alloy and insulator.
- Give one example of each of these materials and state one use of each material in the design of an electrical appliance say an electric stove or an electric iron.
Question: 33.What are decomposers? Give two examples. State how they maintain a balance in an ecosystem.
Section – D
Question Nos. 34 to 36 are long answer type questions. Each question carries 5 marks.
Question: 34. (A)
A carbon compound ‘A’ on heating with excess conc. H2SO, forms a compound ‘B which on addition of one mole of hydrogen gas in the presence of nickel catalyst forms a compound *C’. ‘C’ on combustion in air forms 2 moles of carbon dioxide and 3 moles of water. Identify ‘A’, ‘B’ and ‘C’ and write their structures. Give chemical equations of the reactions involved. Also state the role of concentrated sulphuric acid in the formation of *B’ from ‘A’.
OR
(B)
A carbon compound ‘A’ is widely used as a preservative in pickles and has a molecular formula C,H,O2. This compound reacts with ethanol to form a sweet smelling compound ‘B’.
- Identify the compound ‘A’ and write its structure.
- Write the chemical equation for the reaction of ‘A’ with ethanol to form compound ‘B’. State the role of presence of an acid in the reaction.
- How can we get compound ‘A’ back from ‘B’ ?
- How can ‘A’ be obtained from ethanol ?
- Name the gas produced when compound ‘A’ reacts with washing soda.
Question: 35. (A)
- What is regeneration? Give one example of an organism that shows this process and one organism that does not. Why does regeneration not occur in the latter?
- Water in a pond appears dark green and contains filamentous structures. Name these structures and the method by which they reproduce. Explain the process.
OR
(B)
- Name the part performing following functions in human male reproductive system :
(a) Carries sperm
(b) Production of male gametes
(c) Whose secretion makes the transport of sperms easier
(d) Provide suitable temperature for sperm formation - Write any two characteristics of sperms.
- What are surgical contraceptive methods? Give the side effect caused by this procedure.
Question: 36. (A)
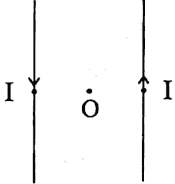
(i) Draw the pattern of the magnetic field lines for the two parallel straight conductors carrying current of same magnitude ‘I’ in opposite directions as shown. Show the direction of magnetic field at a point O which is equidistant from the two conductors. (Consider that the conductors are inserted normal to the plane of a rectangular cardboard.)
(ii) In our houses we receive A.C. electric power of 220 V. In electric iron or electric heater cables having three wires with insulation of three different colours – red, black and green are used to draw current from the mains.
- What are these three different wires called? Name them colour wise.
- What is the potential difference between the red wire and the black wire?
- What is the role of the Wire with green insulation in case of accidental leakage of electric current to the metallic body of an electrical appliance?
OR
(B)
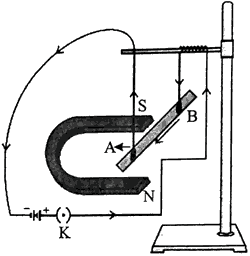
- By using the given experimental set-up. How can it be shown that
(a) a force is exerted on the current-carrying conductor AB when it is placed in a magnetic field.
(b) the direction of force can be reversed in two ways. - When will the magnitude of the force be highest?
- State Fleming’s left hand rule.
Section – E
Question Nos. 37 to 39 are Source-based / Case-based questions.
Question: 37. Common salt is a very important chemical compound for our daily life. Chemical name is sodium chloride and it is used as a raw material in the manufacture of caustic soda, washing soda, baking soda etc. It is also used in the preservation of pickles, butter, meat etc.
(i) Name the acid and the base from which common salt can be obtained.
(ii) State the nature (acidic/basic/neutral) of sodium chloride. Give reason for the justification for your answer.
(iii) (A) What happens when electric current is passed through an aqueous solution of sodium chloride (called brine)? Name the
products obtained along with the corresponding places in the electrolytic cell where each of these products is obtained.
OR
(iii) (B) How is washing soda obtained from sodium chloride? Give a chemical equation of the reactions involved in the process.
Question: 38. In life there are certain changes in the environment called ‘stimuli’ to which we respond appropriately. Touching a flame suddenly is a dangerous situation for us. One way is to think consciously about the possibility of burning and then moving the hand. But our body has been designed in such a way that we save ourself from such situations immediately.
(i) Name the action by which we protect ourself in the situation mentioned above and define it.
(ii) Write the role of (a) motor and (b) relay neuron. 1
(iii) (A) What are the two types of nervous system in the human body? Name the components of each of them. 2
OR
(iii) (B) Which part of the human brain is responsible for : 2
(a) thinking
(b) picking up a pencil
(c) controlling blood pressure
(d) controlling hunger
Question: 39. ‘The students in a class took a thick sheet of cardboard and made a small hole in its center. Sunlight was allowed to fall on this small hole and they obtained a narrow beam of white light. A glass prism was taken and this white light was allowed to fall on one of its faces. The prism was turned slowly until the light that came out of the opposite face of the prism appeared on the nearby screen. They studied this beautiful band of light and concluded that it is a spectrum of white light.
(i) Give any one more instance in which this type of Spectrum is observed.
(ii) What happens to white light in the above case?
(iii) (A) List two conditions necessary to observe a rainbow.
OR
(iii) (B) Draw a ray diagram to show the formation of a rainbow. Mark on it, points (a), (b) and (c) as given below:
(a) Where dispersion of light occurs.
(b) Where light gets reflected internally.
(c) Where final refraction occurs.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students




