Modified Based on Latest CBSE Question Paper Design
Maximum marks: 80
Class: X (Science)
Year: 2012 – 13 (Delhi)
General Instructions:
- The Question paper comprises of two sections, A and B. You are to attempt both the sections.
- All questions compulsory.
- All questions of Section A and all questions of Section B are to be attempted separately.
- Question number 1 to 2 in Section A are one mark questions. These are to be answered in one word or in one sentence.
- Question numbers 3 to 5 in Section A are two marks questions. These are to be answered in about 30 words each.
- Question numbers 6 to 15 in Section A are three marks questions. These are to be answered in about 50 words each.
- Question numbers 16 to 21 in Section A are five marks questions. These are to be answered in about 70 words each.
- Question numbers 22 to 27 in Section B are questions based on practical skills and are two marks question.
Section A
Question: 1. Mention the angle between a current carrying conductor and magnetic field for which the force experienced by this current carrying conductor placed in magnetic field is largest? [1]
Answer: The force is the largest, when angle between the current carrying conductor and magnetic field direction is a right angle, i.e., 90°
Question: 2. Name the sensory receptors found in the nose and on the tongue. [1]
Answer:
- Nose has olfactory receptors which detect smell.
- Tongue has gustatory receptors which detect taste.
Question: 3. Define a solar panel. [2]
Answer:
- A large number of solar cells joined together in a definite pattern is called a solar panel.
- A solar panel can provide much more electric power than a single solar cell.
Question: 4. Write the balanced chemical equation for the following reaction and identify the type of reaction and define it.
‘Iron III oxide reacts with Aluminium and gives molten iron aluminium oxide’. [2]
Answer: Fe2O3(s) → 2Fe (l) + Al2O3(s) + Heat
- It is a displacement reaction which is highly exothermic. The amount of heat evolved is so large that the metal is produced in the molten state.
- The displacement reaction of iron (III) oxide with aluminium is known as thermite reaction.
Question: 5. We often observe domestic waste decomposing in the by-lanes of residential colonies. Suggest ways to make people realize that the improper disposal of waste is harmful to the environment. [2]
Answer:
- When the domestic waste is decomposed by the reaction of micro-organisms then it becomes the breeding place of flies and mosquitoes. Flies and mosquitoes are the carrier and vector of many dangerous diseases. So some posters related to the spread of such diseases can be pasted on the walls in public places to create awareness.
- There should be a strict fine imposed by the sanitary officer from the families which throw their domestic wastes on the roads.
Question: 6. A reddish brown colored metal, used in electrical wires, when powdered and heated strongly in an open china dish, its colour turns black. When hydrogen gas is passed over this back substance, it regains its original colour. Based on the above information answer the following questions:
(i). Name the metal and the black colored substance formed.
(ii). Write balanced chemical equations for both the reactions. [3]
Answer:
(i). The reddish brown colored metal used in electric wires is Copper.
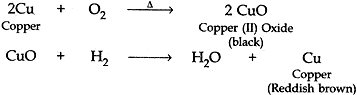
Question: 7.
(a) Give an example for a combination reaction which is exothermic.
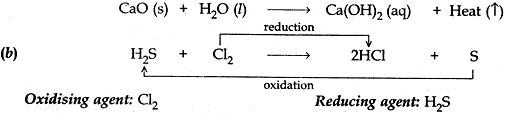
(b). Identify the oxidising agent, reducing agent in the following reaction.
H2S + Cl2 → 2 HCl + S
(c) Name the phenomenon due to which the taste and smell of oily food changes when kept for a long time in open. Suggest one method to prevent it. [3]
Answer: (a) When calcium oxide and water combine to form a single product calcium hydroxide, a large amount of heat is also of heat is also released. Therefore it is a combination reaction which is exothermic.
(c) The phenomenon is rancidity. When fast and oils are oxidised, they become rancid and their smell and taste changes. This Phenomenon is called rancidity.
Prevention from rancidity. Antioxidants are added to food containing fats and oils to prevent them from oxidation. For example, An inert gas such as nitrogen is added to prevent the packed chips from getting oxidised.
Or
(1) Write the name given to based that are highly soluble in water? Give an example.
(2) How is tooth decay related to pH? How can it be prevented?
(3) Why does bee sting cause pain and irritation? Rubbing of baking soda on the sting area gives relief. How?
Answer:
- Water soluble bases are called alkalis, e.g., NaOH, KOH.
- Tooth decay starts when the pH of the mouth is lower than 5.5. Bacteria present in the mouth produce acids by degradation of sugar and food particles remaining in the mouth after eating. This can be prevented by cleaning the mouth and teeth using tooth pastes which are generally basic and can neutralize the excess acid.
- Bee sting leaves an acid which cause pain and irritation. Therefore, to give relief from the pain, a mild base like baking soda is rubbed on the strung area as the mild base neutralizes the acid.
Question: 8.
(1) Why does calcium start floating when added to water?
(2) Most of the metals do not give hydrogen while reacting with nitric acid. Why?
(3) Write equation for the reaction of iron with steam. Name the compound of iron obtained.
Answer:
- The reaction of the calcium with water is less violent. The heat evolved is not sufficient for hydrogen to catch fire.
Ca (s) + 2H2O(l) → Ca(OH)2 (aq) + H2 (g)
Calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of the metal. - Most of the metals do not give hydrogen reacting with nitric acid because HNO3 is a strong oxidising agent. It oxidises the H2 produced to water (H2O) and itself gets reduced to any of the nitrogen oxide and (N2O, NO, NO2).
- Iron reacts with steam to form iron oxide and hydrogen
3Fe (s) + 4H2O (g) → Fe3O4(s) + 4H2 (g)
Question: 9. Three resistors of 5 Ω, 10 Ω and 10 Ω are connected in series and the combination is connected to battery of 30 V. Ammeter and Voltmeter are connected in the circuit. Draw a circuit diagram to connect all the devices in proper correct order. What is the current flowing and potential difference across 10 Ω resistance? [3]
Answer: Current flowing, I = ?, V2 = ?
Total resistance, R = R2 + R2 + R3
= 5 + 10 + 15 = 30 Ω
Total potential difference, V = 30 volts
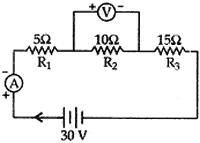
According to Ohm’s law
V = IR ⇒ I = V/R = 30/30 = 1 amp.
Current remains constant in series
∴ I1 = I2 = I3 I2 = 1 amp R2 = 10 Ω V2 = ?
As V2 = I2R2 = 1 × 10 = 10 volts
∴ Potential different across 10 Ω is 10 volts.
Question: 10.
(1). Name the part of brain which controls
(i) voluntary action, (ii) involuntary action.
(2). What is the significance of the peripheral nervous system? Name the components of this nervous system and distinguish between the origin of the two. [3]
Answer:
(a). (i) All the voluntary actions of the body are coordinated by the Cerebellum.
(ii) Various involuntary actions are controlled by Medulla.
(b). Peripheral nervous system is extended between the central nervous system and body parts. The communication between the central nervous system and the other parts of the body is facilitated by the peripheral nervous system. On the basis of their origin, it is divided into two types of nerves.
(i) Cranial nerves. These nerves extend between the brain and parts of head except 10th cranial nerves which extends up to the abdomen.
(ii) Spinal nerves. There nerves extend between spinal cord and the body parts.
| Character | Cranial nerves | Spinal nerves |
| 1. Extension 2. Number 3. Nature |
Between brain and body parts. 12 pairs May be sensory or motor or mixed |
Between spinal cord and body parts. 31 pairs Always mixed. |
Question: 11. Why homologous series of carbon compounds are so called? Write chemical formula of two consecutive members of a homologous series and state the part of these compounds that determines their (i) physical properties, and (ii) chemical properties. [3]
Answer:
- All the organic compounds having similar structures show similar properties and they are put together in the same groups or series called homologous series.
- For example, all the alkanes have similar structures with single covalent bonds and show similar chemical properties so they are grouped together in a homologous series having general formula CnH2n+2.
- The two consecutive members of a homologous series are methane CH4 and ethane C2H6.
(i) any two adjective homologous differ by 1 carbon and 2 hydrogen atoms in their molecular formulae. So the difference in molecular masses of any two adjacent homologous is 14 u. Thus the members of a homologous series show a gradual change in their physical properties with increase in molecular mass.
(ii) All the compounds of a homologous series show similar chemical properties because they have similar structures and similar bonding.
Question: 12. Write two examples each of sexually transmitted diseases caused by (i) virus, (ii) bacteria. Explain how the transmission of such diseases be prevented?
Answer: Sexually transmitted diseases caused by
(i) Virus: (1) AIDS (Acquired Immuno Deficiency Syndrome); (2) Genital warts
(ii) Bacteria: (1) Gonorrhoea; (2) Syphilis
Transmission of such diseases can be prevented by the following ways:
- Screening tests for blood donors
- Mutually faithful monogamous relationships
- Educating people in high risk groups
- Using condoms, etc.
Question: 13. “The sex of a newborn child is a matter of chance and none of the parents may be considered responsible for it.” Justify this statement with the help of a flow chart showing determination of sex of a newborn. [3]
Answer: Sex determination flow chart:
- A male has one X-chromosome and Y-chromosome. Thus half the male gametes have X-chromosomes and the other half have Y-chromosomes.
- A female has two X-chromosomes. Thus all female gametes have only X-chromosomes.
- If a sperm carrying Y-chromosome fertilises an ovum carrying X-chromosome, then the child born will be a boy.
- If a sperm carrying X-chromosome fertilises and ovum carrying X-chromosome, then the child born will be a girl.

Therefore it is the sperm from the father which determines the sex of the child.
Thus in human beings, the sex of baby is determined by the type of sperm that fuses with ovum. As human male produces two types of sperms in equal proportion, so there are 50% chances of a male baby and 50% chances of a female baby.
Question: 14. Mention the types of mirrors used as (i) rear view mirrors, (ii) shaving mirrors. List two reasons to justify your answers in each case. [3]
Answer:
- Convex mirror is used as rear view mirror in vehicles because
(i). it always produces an erect image of the objects;
(ii). the image formed in a convex mirror is highly diminished thus it gives a wide field of view. - Concave mirrors are used as shaving mirrors because
(i). when the face is held within the focus of a concave mirror, than an enlarged image of the face is seen in the concave mirror. This helps in making a smooth shave.
Or
An object of height 6 cm is placed perpendicular to the principal axis of a concave lens of focal length 5 cm. Use lens formula to determine the position, size and nature of the image if the distance of the object from the lens is 10 cm.
Sol. Height of the object, h1 = 6 cm
Focal length of the concave mirror, f = -5 cm
Position of the image, v = ? Size of the image, h2 = ?
Object distance, u = -10
According to lens formula:
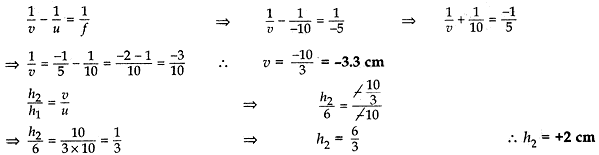
Thus the image is formed at a distance or 3.3 cm from the concave lens. The negative (-) sign for image distance shows that the image is formed on the left side of the concave lens (i.e., virtual). The size of the image is 2 cm and the positive (+) sign for hand image shows that the image is erect.
Thus a virtual, erect, diminished image is formed on the same side of the object (i.e., left side).
Question: 15. State the difference in colors of the Sun observed during sunrise/sunset and noon. Give explanation for each. [3]
Answer: The Sun and surrounding sky appear red at sunrise and at sunset because at this time the Sun is near the horizon and sunlight has to travel the greatest distance through the atmosphere to reach us. Thus most of the blue colour present in sunlight has been scattered out and away from our line of sight, leaving behind mainly red colour in the direct sunlight beam that reaches our eyes.
When the Sun is overhead (as at noon) then the light coming from the Sun has to travel a relatively shorter distance through the atmosphere to reach us. Thus only a little of blue colour of the white light is scattered. Since the light coming from the overhead Sun has almost all its component colours in the right proportion, therefore the Sun in the sky overhead appears white.
Question: 16. (a). In the formation of compound between two atoms A and B, A loses two electrons and B gains one electron.
(i) What is the nature of bond between A and B?
(ii) Suggest the formula of the compound formed between A and B.
(b). On similar lines explain the formation of MgCl2 molecule.
(c). Common salt conducts electricity only in the molten state. Why?
(d). Why is melting point of NaCl high? [5]
Answer: (a)
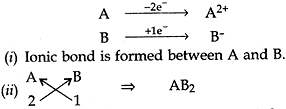
(b) (i) Mg has 2 election in its outermost shell so it loses 2 electrons to achieve the inert gas configuration of eight valence electrons and forms positively charged ion or divalent cation.
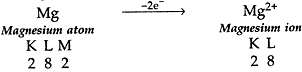
(ii) Cl has 7 electrons in its outermost shell so it gains one electron to achieve the stable inert gas configuration and forms negatively charged ion or monovalent anion.
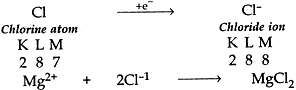
(c) Common salt (NaCl) is an ionic compound which conducts electricity only in molten state because in molten state the electrostatic forces of attraction between oppositely charged ions (Na+ and Cl–) are overcome due to heat. Thus the ions move freely and conduct electricity.
(d) NaCl is an ionic compound so there is a strong force of attraction between the positively charged sodium ion and negatively charged chloride ion. Therefore a considerable amount of energy is required to break the strong interionic attraction. Thus NaCl has high melting point.
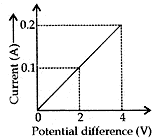
Question: 17. (a). Calculate the resistance of the wire using the graph. [5]
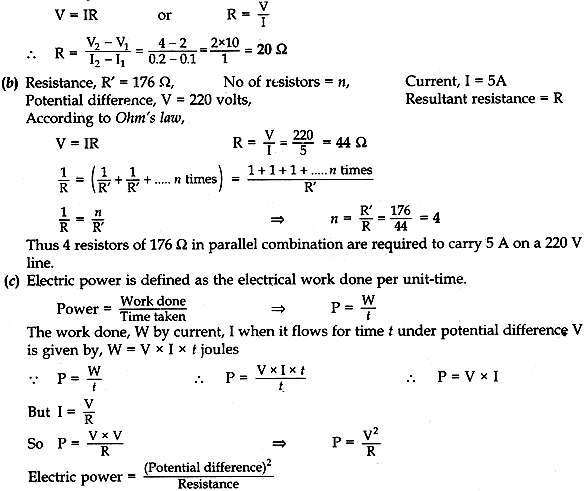
(b) How many 176 Ω resistors in parallel are required to carry 5 A on a 220 V line?
(c) Define electric power. Derive relation between power, potential difference and resistance.
Answer: (a) Resistance of wire = Slope of the graph
According to Ohm’s law,
Question: 18. Draw the diagram of sectional view of human heart and on it name label the following parts:
(1) The chamber of the heart that pumps out deoxygenated blood.
(2) The blood vessel that carries away oxygenated blood from the heart.
(3) The blood vessel that receives deoxygenated blood from the lower part of our body. [5]
Answer:
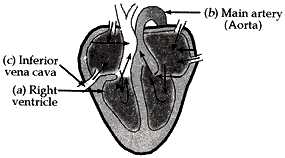
- The chamber of the heart that pumps out deoxygenated blood – Right ventricle
- The blood vessel that carries away oxygenated blood from the heart – Aorta
- The blood vessel that receives deoxygenated blood from the lower part of our body – Inferior Vena Cave
Question: 19. (1) List three distinguishing features between sexual and asexual types of reproduction
(2) Explain why variations are observed in the offsprings of sexually reproducing organisms? [5]
Answer:
- Asexual reproduction:
(i) Only one organism is required.
(ii) New organism produced is genetically similar to the parent organism.
(iii) All division involved are mitotic.
(iv) It dos not help in evolution.
Sexual reproduction:
(i) Two separate individuals, male and female are required.
(ii) New organism produced is genetically different from both.
(iii) During gamete formation meiosis occurs. After fertilization, all divisions are mitotic.
(iv) It helps in evolution. - Reason. There is always a possibility of diversity of characters in the offspring because is formed as a result of fusion of two gametes produced by two different individuals – the male and the female parents. So there is an opportunity for new combinations of characters.
Or
(1) Identify A, B and C in the given diagram and write their functions.
(2) Mention the role of gamete and zygote in sexually reproducing organisms.
Answer: (1).
A – Stigma. The top part of carpel is called stigma. Stigma is for receiving the pollen grains from the anther of stamen during pollination.
B – Pollen tube. When a pollen grain falls on the stigma, it bursts open and grows a pollen tube downward through the style towards the female gamete in the ovary. A male gamete moves down the pollen tube.
C – Female gamete (ovum). It is a special reproductive female sex cell which combines with male gamete to form zygote.
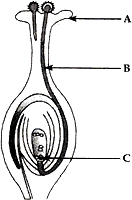
(2). Sexual reproduction takes place by the combination of special reproductive cells called sex cells. These cells are of two types: male sex cells and female sex cells, which are coming from two different parents: a male and a female. The cells involved fuses with a female gamete to form a new cell called zygote. This zygote then grows and develops into a new organism in due course of time.
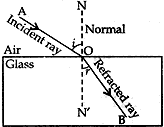
Question: 20. (a) State the lows of refraction of light. Give an expression to relate the absolute refractive index of a medium with speed of light in vacuum.
(b) The refractive indices of water and glass is 2 × 108ms-1, find the speed of light in (i) air, (ii) water. [5]
Answer: (a) There are two laws of refraction of light.
(i). The first law of refraction of light states that the incident ray, the refracted ray and the normal at the point of incidence, all lie in the same plane.
(ii) The second law of refraction of light is the Snell’s law of Refraction. It states that the ratio of sine of the of angle of refraction is a constant for a given pair of medium.
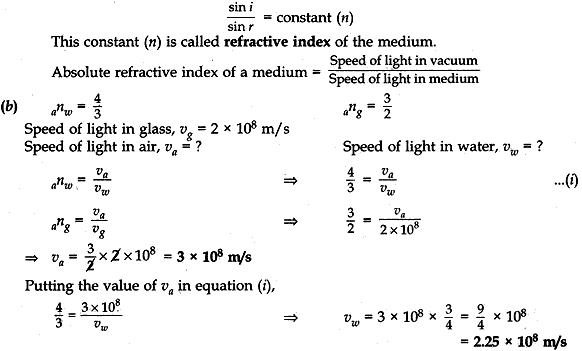
Question: 21. The elements of the third period of the Periodic Table are given below:
Group → I II III IV V VI VII
Period ↓ Na Mg Al Si P S Cl
(1) Which atom is bigger, Na or Mg? Why?
(2) Identify the most (i) metallic and (ii) non-metallic element in period 3.
(3) Which is more non-metallic, S or Cl?
(4) Which has higher atomic mass, Al or Cl? [5]
Answer:
- Na is bigger than because on moving from left to right in a period, the atomic number of elements increases which means that the number of protons and electrons in the atom increases. (The extra elections beign added to the same shell).
- (i) Most metallic element is Na. Most non-metallic element is cl;
Because on moving from left to right in a period the nuclear charge increases thus the valence electrons are pulled in more strongly by the nucleaus and it becomes more and difficult for the atoms to lose electrons so tendency of atoms to lose electrons (i.e., metallic character) decreases on moving from left to right in a period. On the other hand, due to increased nuclear charge, it becomes easier for the atoms to gain electrons. So the tendency to gain electrons (i.e., non-metallic character) increases on moving from left to right in a period. - Cl is more non-metallic from S because on moving from to right in a period, nuclear charge increases so the tendency to gain electrons increases (i.e., non-metallic character).
- Cl has higher atomic mass because on moving from left to right in a period, atomic number increases. Simultaneously, atomic mass increases.
Section B
Question: 22. What is of inertial is formed when aqueous solutions of sodium sulphate and barium chloride are mixed. Give the balanced chemical equation involved. Name the type of reaction it is? [2]
Answer:
- A white precipitate of BaSO4 will be formed.
- Na2SO4 (aq) + BaCl2 (aq) → BaSO4 + 2NaCl (aq)
- It is a double displacement reaction.
Question: 23. Give any four precautions taken by a student to perform an experiment to determine the resultant resistance of two resistors when connected in series. [2]
Answer:
- Clean the ends of the connecting wires by sand paper.
- The connections should be tight.
- Close the key (K) only when readings are to be taken.
- Zero errors and the ranges of the ammeter and voltmeter should be noted.
Question: 24. Why is epidermal peel generally taken from lower surface of the leaf? [2]
Answer: In dicot plants stomata are found in lower surface only, hence peel is taken from the lower surface. As stomata are present on both the surface only of leaf in monocot plants so the peel can be taken from either surface.
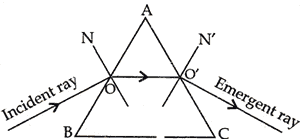
Question: 25. Why does a ray of light while passing through a prism, bend towards its base? [2]
Answer: When a ray of light passes through a prism, it under goes refraction twice. First from rarer to denser medium of glass, it bends towards normal which is towards the base of the prism. Second time from denser to rarer medium, i.e., glass to air, it bends away from the normal, i.e., towards the base of the prism.
Question: 26. Name two types of fissions. Name two living beings of each type which reproduce by these methods of fission. [2]
Answer: Two types of fissions are: (i) Binary Fission, (ii) Multiple Fission
Two examples of binary fission – Amoeba and Paramoecium
Two types of multiple fission are – Plasmodium and Spirogyra
Question: 27. Write two tests you would perform to detect, whether the given colorless liquid is Acetis Acid or not. [2]
Answer: Two tests:
- If we put a drop of the given colorless liquid on blue litmus paper, if the blue litmus paper changes to red, then the given acid is Acetic acid.
- If we smell the given liquid and the gives a smell like that of vinegar, then the given acid is Acetic acid.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students