10th CBSE Science Post Mid-Term Exam 2018-19
Time: 3 hours
Maximum Marks: 80
Class: 10th
Date: ?? / ?? / 2018/19
Subject: Science
General Instructions:
- All questions are compulsory. However there is an internal choice in one question of two marks, three question of three marks each three questions of five marks each and 3 questions of practical based question.
- Question numbers 1 and 2 are one mark question.
- Question numbers 3 to 5 are two marks questions.
- Question numbers 6 to 15 are three marks questions.
- Question numbers 16 to 21 are five marks questions.
- Question numbers 22 to 27 are two marks questions.
10th CBSE Science Question Paper: Section A
Question: 1. Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example.
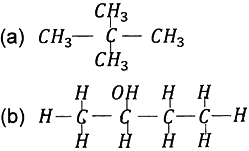
Question: 2. Write the molecular formula of the 2nd and 3rd member of the homologous series whose first member is methane.
10th CBSE Science Post Mid-Term Exam: Section B
Question: 3. After rains, during day time a rainbow often appears in the sky. Explain how this natural phenomenon occurs, with the help of a diagram.
Question: 4. (i) “Forests are biodiversity hotspots” Give reason to justify this statement.
(ii) State the meaning of ‘Sustainable Development’.
Question: 5. Which natural resources are the ‘biodiversity hotspots’? Suggest what happens when there is a loss of biodiversity.
OR
Quote two instances where human intervention saved the forests from destruction.
Section C
Question: 6. 2g of lead nitrate powder is taken in a boiling tube. The boiling tube is heated over a flame. Now answer the following:
- State the colour of the, fumes evolved and the residue left.
- Name the type of chemical reaction that has taken place stating its balanced chemical equation.
Question: 7. Tow carbon compounds X and Y have the molecular formula C4H8 and C5H12 espectively. Which one of these is most likely to show addition reaction? Justify your answer. Also, give the chemical equation to explain the process of addition reaction in this case.
Question: 8. Write the name and molecular formula of an organic compound having its name suffixed with ‘ol and having two carbon atoms in the molecule. With the help of a balanced equation indicate what happens when it is heated with excess of conc. H2SO4.
OR
- Name the following:
- Third member of aldehyde series.
- Second member of carboxylic series.
(ii) Write the IUPAC name of the following:
Question: 9. Draw a diagram of human brain and label on it cerebrum and cerebellum State the role of cerebellum.
Question: 10. Explain how the human body responds when adrenaline is secreted into the blood.
Question: 11. An environmentalist on visit to your school suggested the use of three R’s to save the environment. Explain what he meant by three R’s and how would you follow his advice-at home.
OR
Management of forest and wildlife resources is a very challenging task. Why? Give any three reasons.
Question: 12. What is colour-blindness? What kind of retinal cells are lacking in person suffering from this defect?
OR
- Why does the sun appear reddish early in the morning?
- Why does the sky appear dark instead of blue to an astronaut.
Question: 13. Describe with the help of a diagram, an activity to show that a current carrying wire behaves like a magnet .
Question: 14. Explain briefly two different ways to induce current in a coil. State the rule which determines the direction of induced current.
Question: 15. What is meant by nuclear waste? State the main hazard of this waste on the living beings. How is this waste disposed off?
10th CBSE Science Post Mid-Term Exam: Section D
Question: 16.
- Stu he following chemical equation:
Name the reactant and the product. State one use of the product and the reactant.
- State in tabular form the name of the acid and base from which the following salts are formed. Also mention the nature of the salt, whether it is acidic or basic.
(a) Sodium acetate.
(b) Ammonium sulphate.
OR
- Explain the following chemical properties of acids with the help of balanced chemical reactions only.
(a) When an acid reacts with a metal carbonate.
(b) When an acid reacts with a metal bicarbonate.
(c) When an acid reacts with a metal oxide.
(ii) You are given three solutions A, B and C with pH values 2, 10 and 13 respectively. Write which solution has more hydrogen ion concentration among the three and state the nature ‘acidic or basic’ of each solution.
Question: 17.
- (a)State the Modern Periodic Law.
(b) What is the total number of periods and groups in Modern Periodic Table. - Na, Mg, and Al are the elements of the 3rd Period of the Modem periodic Table having group number 1,2 and 13 respectively. Which one of these elements has the (a) highest valency, (b) largest atomic radius and (c) maximum chemical reactivity. Justify your answer stating the reason for each.
Question: 18.
- Draw the diagram of sectional view of human heart and label on it:
(a) Chamber which receives deoxygenated blood from vena ceva.
(b) Chamber which pumps oxygenated blood into Aorta.
(c) Blood vessel which brings oxygenated blood to the heart.
(d) Chamber which receives oxygenated blood from lungs. - What is double circulation of blood?
OR
- Draw a diagram of human alimentary canal and label on it.
(a) Organ which stores bile.
(b) Organ which produces bile. - Name one enzyme present in pancreatic juice and give its functions.
- What are peristaltic movements?
Question: 19.
- Explain why does menstruation occur in human females.
- (a) Mention two functions of the ovaries in human female reproductive system.
(b) Mention the name of organ.
(A) That provides nutrition to embryo.
(B) site where fertilized egg gets implanted.
Question: 20.
- The absolute refractive index of water is 4/3. What is meant by this statement?
- A pencil partially immersed in water appears to be bent. Name the phenomenon responsible for this observation and explain it on its basis.
- An object is placed at a distance of 25m from a concave mirror of focal length 10m. Find the nature and position of the image so formed.
OR
- How can you distinguish between a plane mirror, concave mirror & convex mirror without touching them?
- An electric bulb is rated 220V and 100W. Calculate the power consumed when it is operated on 110V.
Question: 21.
- What is heating effect of current? List two electrical appliances which work on this effect.
- State the-difference between kilowatt and kilowatt hour.
- Given that R1 = 10 Ω, R2 = 40 Ω, R3 = 30 Ω, R4 = 20 Ω and RA is the parallel combination of R1 and R2. Whereas RB is the parallel combination of R3 & R4. Combination RA is connected to the positive terminal of 12V battery while RB is connected to the negative terminal. Ammeter A is connected to the negative terminal. Ammeter A is connected between the resistors RA and RB. Find RA and RB. Also calculate the total resistance in the circuit.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students