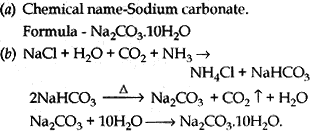
Question: (a) Write the chemical name and chemical formula of washing soda.
(b) How is it obtained from sodium chloride? Give equations of the reactions.
(c) Why is it called a basis salt? Give its any one use.
Answer:
(c) It is a basic salt because when dissolves in water it gives a strong base NaOH.
(i) In glass, paper industry.
(ii) As cleaning agent.
(iii) For removing permanent hardness. (any one)
Question: (a) Identify the acid and the base whose combination forms the common salt that you use in your food. Write its formula and chemical name of this salt. Name the source from where it is obtained.
(b) What is rock salt? Mention its colour and the reason due to which it has this colour.
(c) What happens when electricity is passed through brine? Write the chemical equation for it.
Answer: (a) Hydrochloric acid and sodium hydroxide. NaCl, sodium chloride, ocean water.
(b) Deposits of solid salt are found in several parts of the world. These large crystals are called rock salt.
Brown, due to impurities.
Question: (1) Tooth enamel is one of the hardest substance in our body. Explain the changes in pH of the mouth which indicate tooth decay. How does tooth paste help in preventing it?
(2) What is the nature of the salt if the pH of its solution is greater than 7? Name the acid and based would be used to prepare the following salts:
(i) Potassium sulphate.
(ii) Ammonium chloride.
Answer:
- Tooth decay starts when pH of the mouth goes below 5.5. This happens when bacteria present in the mouth produce acids by degradation of sugar and food particles remaining in the mouth after eating using toothpaste which are generally basic.
- Basic salt:
(i) Base – KOH (potassium hydroxide).
Acid – H2SO4 (sulphuric acid)
(ii) Base – NH4OH (ammonium hydroxide).
Acid – HCl (hydrochloric acid)
Question: (1) Study the following chemical equation:
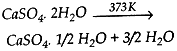
Name the reactant and the product. State one use of the product.
(2) State in tabular form the same of the acid and base from which following salts are formed. Also, mention the nature of the salt, whether it is acidic or basic?
(i) Sodium acetate.
(ii) Ammonium sulphate.
Answer:
- Reactant – Gypsum
Product – Plaster of Paris
Use – Healing the fractured bones / casting moulds for statues or toys / dentistry. - SaltAcidBaseNatureSodium acetateAcetic acidSodium hydroxideBasicAmmonium sulphateSulphuric acidAmmonium hydroxideAcidic
Question: (a) A salt is produced by the reaction between an acid and a base. Identify the acid and the base from which the following salts have been formed:
(i) Na2SO4 (ii) NH4Cl (iii) KNO3 (iv) NaCl
(b) Which of the above salts has a pH less than 7? Why?
Answer: (a)
(ii) NH4Cl
(iii) KNO3
(iv) NaCl
HCl
NHO3
HCl
NH4Cl
KOH
NaOH
(b) NH4Cl will have pH less than 7, because it is formed from strong acid (which ionises completely) and weak base (which does not ionises completely).
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students