Acids, Bases and Salts MCQs: CBSE Class 10 Science Chapter 2 Acids, Bases and Salts Multiple Choice Questions with Answers. MCQ Questions for Class 10 Science with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 10 Science Acids, Bases and Salts Multiple Choice Questions with Answers to know their preparation level.
| Class: | 10th Class |
| Subject: | Science |
| Chapter: | Chapter 02: Acids, Bases and Salts |
| Quiz: | 37 Questions |
Acids, Bases and Salts MCQs
MCQs for Class 10 Science Acids, Bases and Salts with Answers & Explanation:
1. What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) Temperature of the solution decreases
(ii) Temperature of the solution increases
(in) Temperature of the solution remains the same
(iv) Salt formation takes place
(a) (i) and (iv)
(b) (i) and (iii)
(c) (ii) only
(d) (ii) and (iv)
Answer 1.
2. When hydrogen chloride gas is prepared on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
(a) absorb the evolved gas
(b) moisten the gas
(c) absorb moisture from the gas
(d) absorb Cl– ions from the evolved gas
Answer 2.
Explanation: Reason: Guard tube drys (absorbs water) from calcium chloride on a humid day.
3. Which one of the following salts does not contain water of crystallization?
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer 3.
4. In terms of acidic strength, which one of the following is in the correct increasing order?
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic acid
Answer 4.
Acids, Bases and Salts MCQs – 5. What is formed when zinc reacts with sodium hydroxide?
(a) Zinc hydroxide and sodium
(b) Sodium zincate and hydrogen gas
(c) Sodium zinc-oxide and hydrogen gas
(d) Sodium zincate and water
Answer 5.
Explanation: Reason: Zn + 2NaOH → Ma2Zn02 (Sodium Zincate) + H2
6. Tomato is a natural source of which acid?
(a) Acetic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
Answer 6.
7. Brine is an
(a) aqueous solution of sodium hydroxide
(b) aqueous solution of sodium carbonate
(c) aqueous solution of sodium chloride
(d) aqueous solution of sodium bicarbonate
Answer 7.
8. Na2CO3 . 10H2O is
(a) washing soda
(b) baking soda
(c) bleaching powder
(d) tartaric acid
Answer 8.
9. At what temperature is gypsum heated to form Plaster of Paris?
(a) 90°C
(b) 100°C
(c) 110°C
(d) 120°C
Answer 9.
Acids, Bases and Salts MCQs – 10. How many water molecules does hydrated calcium sulphate contain?
(a) 5
(b) 10
(c) 7
(d) 2
Answer 10.
Explanation: Reason: Chemical formula of hydrated calcium sulphate or gypsum is CaSO4.2H2O
Acids Bases and Salts MCQs: 10th Science Chapter 2
11. Sodium carbonate is a basic salt because it is a salt of a
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Answer 11.
12. Alkalis are
(a) acids, which are soluble in water
(b) acids, which are insoluble in water
(c) bases, which are insoluble in water
(d) bases, which are soluble in water
Answer 12.
13. Which of the following statements is correct about an aqueous solution of an acid and of a base?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(in) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer 13.
Explanation: Reason: Stronger the acid, lesser is the pH. Stronger the base, higher is the pH.
14. The apparatus given in the adjoining figure was set up to demonstrate electrical conductivity.
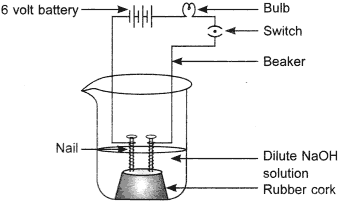
Which of the following statement(s) is (are) correct?
(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because HCl is a strong acid and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) only
Answer 14.
Acids, Bases and Salts MCQs – 15. Lime water reacts with chlorine to give
(a) bleaching powder
(b) baking powder
(c) baking soda
(d) washing soda
Answer 15.
Explanation: Reason:
![]()
16. Nettle sting is a natural source of which acid?
(a) MetiWanoic acid
(b) Lactic acid
(c) Citric acid
(d) Tartaric acid
Answer 16.
17. Tooth enamel is made up of
(a) calcium phosphate
(b) calcium carbonate
(c) calcium oxide
(d) potassium
Answer 17.
18. What is the pH range of our body?
(a) 7.0 – 7.8
(b) 7.2 – 8.0
(c) 7.0 – 8.4
(d) 7.2 – 8.4
Answer 18.
19. Rain is called acid rain when its:
(a) pH falls below 7
(b) pH falls below 6
(c) pH falls below 5.6
(d) pH is above 7
Answer 19.
Acids, Bases and Salts MCQs – 20. Sodium hydroxide is a
(a) weak base
(b) weak acid
(c) strong base
(d) strong acid
Answer 20.
Explanation: Reason: Sodium hydroxide ionizes in water and produces a large amount of hydroxide ions.
Acids Bases and Salts MCQs: 10th Science Chapter 2
21. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking powder
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Answer 21.
22. When copper oxide and dilute hydrochloric acid react, colour changes to
(a) white
(b) bluish-green
(c) blue-black
(d) black
Answer 22.
Explanation: Reason: Blue-green colour of solution is due to the formation of copper (II) chloride.
23. Sodium hydroxide is used
(a) as an antacid
(b) in manufacture of soap
(c) as a cleansing agent
(d) in alkaline batteries
Answer 23.
24. Sodium hydroxide turns phenolphthalein solution
(a) pink
(b) yellow
(c) colorless
(d) orange
Answer 24.
25. Chemical formula of washing soda is
(a) Na2C03 . 7H2O
(b) Na2C03 . 5H2O
(c) Na2C03 . 2H2O
(d) Na2C03 . 10H2O
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students





