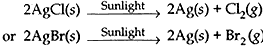Question: Describe an activity to show a decomposition reaction inn which light is used to decompose a reactant. Write chemical equation of the reaction and state its one use.
Answer:
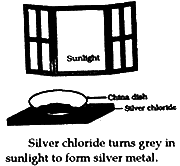
- Take 2g silver chloride in a china dish.
- Place this china dish in sunlight for sometime.
- Observe the colour of silver chloride after sometime. Silver chloride after sometime. Silver chloride turns grey in sunlight due to decomposition of AgCl to Ag and Cl2.
Question: 2 g of ferrous sulphate crystals are heated in a boiling tube.
(i). State the colour of ferrous sulphate crystals both before heating and after heating.
(ii). Name the gases produced during heating.
(iii). Write the chemical equation for the reaction.
Answer:
(i). Before heating – Pale green. After heating – Brown or reddish brown.
(ii). SO2 and So3.
Question: Give reasons for the following:
(1). All decomposition reactions are endothermic reactions.
(2). Color of copper sulphate solution changes when an iron nail is dipped in it.
(3). Respiration is an exothermic reaction.
Answer:
- Decomposition reactions require energy either in the form of heat, light or electricity for breaking down the reactants or energy is absorbed.
- Iron has displaced copper from copper sulphate solution to form iron sulphate which is light green in colour. Fe is more reactive than copper.
- During digestion, food (rice, potatoes etc.) containing carbohydrates are broken down to form glucose. This glucose combines with oxygen in the cells of our body and provided energy. Since energy is given so it is an exothermic.
Question:
(1) Define corrosion.
(2) What is corrosion of iron called?
(3) How will you recognize the corrosion of silver?
(4) Why corrosion of iron is a serious problem?
(5) How can we prevent corrosion?
Answer:
- Corrosion: The process in which metals breakdown gradually by the action of air, moisture or a chemical on their surface.
- Rusting of iron.
- By the development of a black coating on silver.
- Every year enormous amount of money is spent to replace damaged iron.
- paint, galvanization, electroplating. (any one)
Question:
(a) Balance the chemical equation: Fe(s) + H2O(g) → Fe3O4(s) → H2(g)
(b) Identify the type of reaction in the equation given below:
Na2SO4(aq) + BaCl2(aq) → BasO4(s) + NaCl(aq)
(c) You could have noted that when copper powder is heated in a china dish, the surface of copper powder becomes coated with black colour substance.
(i) Why has this black coloured substance formed?
(ii) What is this black substance?
(iii) Write the chemical equation of the reaction that takes place.
Answer: (a) 3Fe(s) + 4H2O(g) → Fe3O4(s) → 4H2(g).
(b) Double displacement reaction.
(c) (i) Black coloured substance is copper oxide. It is formed because oxygen is added to copper.
(ii) Copper (II) oxide (CuO).
![]()
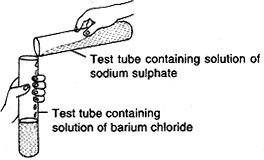
Question: Observe the given figure and water the following questions:
- Write the complete balanced reaction for the above.
- Write type of reaction involved.
- Is there any precipitate formed?
- If any precipitate formed, write the colour of the precipitate.
Answer:
- Na2SO4(aq) + BaCl2(aq) → 2NaCl(aq) + BaSO4(s)↓ (White precipitate)
- Double displacement reaction.
- Yes.
- White precipitates of barium sulphate are formed.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students