Question: A compound ‘Z’ is formed by the transfer of electrons from a metals ‘X’ to a non-metals ‘Y’. Identify the type of bond formed of the compounds formed by such type of bonds.
Answer: Ionic compounds are formed in these compounds. The general properties of ionic compounds are:
- Solid
- High melting and boiling point
- Soluble in water
- Goods conductor of electricity
Question: Give reason for the following:
-
School bells are made-up of metals.
-
Electrical wires are made-up of copper.
Answer:
- Metals are sonorous, so school bells are made-up of metals.
- Copper is a very good conductor of electricity. So, it is used for making electric wires.
Question: Out of the metals P and Q, P is less reactive than Q. Suggest an activity to arrange these metals in the order of their decreasing reactivity. Support your answer with a suitable chemical equation.
Answer: In a test tube, a small amount of salt solution of P is taken and metal Q is added into it. Q being more reactive, displaces metal P from its salt solution.
Chemical equation:
Metal Q + Salt solution of P → Salt solution of Q + Metal P.
Question: Define an alloy. How is an alloy prepared?
Answer: Homogeneous mixture of two or more metals of a metals and a non-metal is known as an alloy. It is prepared by first melting the primary metal, and then, dissolving the other elements is it in definite proportions.
Question: Give reason:
-
Sodium metal is stored under kerosene oil.
- Inspite of being highly reactive, aluminium is still used for making utensils.
Answer:
- They react so vigorously (in the presence of moisture) that they catch fire if kept in open.
- Because its surface gets covered with a protective oxide layer which prevents the metal from further oxidation. Moreover, this oxide layer does not get removed even on heating.
Question: What happens to potassium and sodium if they are kept in open? Why they are immersed is kerosene?
Answer: They react so vigorously that they catch fire if kept in the open. Hence, to protect them and to prevent accidental fires, they are kept immersed in kerosene oil.
Question: Why hydrogen gas is not evolved when a metal reacts with nitric acid?
Answer: It is because nitric acid (HNO3) is a strong oxidizing agent. It oxidizes the H2 Produced to water and itself get reduced to any of the nitrogen oxides (N2O, NO, NO2).
Question: Why is iron galvanized with zinc? Can it be galvanized with zinc? Can it be galvanized with copper? If not, why?
Answer: Iron is galvanized with zinc to protect it from rusting. This is because zinc is more electropositive than iron. Copper is less electropositive than iron. Therefore, iron cannot be galvanized with copper.
Question: Differentiate between roasting and calcination.
Answer: Roasting:
- In this the ore is heated in the presence of excess of air.
- It is done for sulphide ores.
- SO2 gas is evolved.
Calcination:
- In this the ore is heated in the absence of excess of air.
- It is done for carbonate ores.
- CO2 gas is evolved.
Question: Name the ore of mercury. How mercury is extracted from its ore?
Answer: HgS – Cinnabar
First, HgS is converted into HgO
![]()
Then, HgO is reduced to mercury on further heating
![]()
Question: How is copper obtained from Cu2S? Give reactions.
Answer: Cu is obtained from Cu2S by just heating in air.
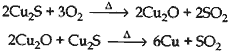
Question: Give reason for the following:
(1). Metals conduct electricity.
(2). Non-metals in general do not displace hydrogen from dilute acids.
(3). Aluminium easily combines with oxygen but still it can be used for making kitchen utensils.
Answer:
- Metals have electrons which are free to move.
- A non-metal is an electron acceptor. It cannot supply electrons to H+ ions. Hydrogen can only be displaced from dilute acids if electrons are supplied to the H+ ions of the acid.
- The layer of aluminium oxide formed prevents the metal from further oxidation.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students