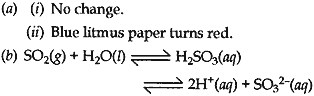Question: Account for the following:
(1) Aluminium is more reactive than iron. But its corrosion is less than iron.
(2) Hydrogen gas is not evolved when zinc metal reacts with dil. HNO3.
(3) Carbon is not used for reducing aluminium from aluminium oxide.
Answer:
- Aluminium is covered with a strong protective layer of oxide which does not peel off easily and thus, protect the metal from further corrosion.
- Nitric acid is a strong oxidising agent. It oxidises the hydrogen produced to water and itself gets reduced to any of the oxide of nitrogen.
- Because aluminium has greater affinity for oxygen than for carbon, i.e., carbon cannot reduce alumina (Al2O3) to aluminium.
Question: (1) Why calcium starts floating when added to water?
(2) Most of the metals do not give hydrogen while reacting with nitric acid Why?
(3) Write equation for the reaction of iron with steam. Name the compound of iron obtained.
Answer:
- Calcium starts floating when added to water because the bubbles of hydrogen gas formed stick to the surface of the metal and makes it lighter.
- HNO3 is a strong oxidising agent. It oxidises H2 to H2O.
- 3Fe + 4H2O → Fe3O4 + 4H2
The compound is ferroferric oxide (Fe3O4).
Question: Define alloys. List the properties of alloys that makes them useful over pure metals. Explain this fact with suitable examples.
Answer: Alloys are homogeneous mixture of two or more metals or a metal and a non-metal that cannot be separated into their components by physical methods.
(i) The electrical conductivity, and
(ii) Melting point of an alloy is less than that of pure metal.
e.g., (a) Brass and bronze (an alloy of Cu) are not good conductors of electricity, whereas copper is used in making electrical circuit.
(b) Solder has a low melting point.
Question: What are amphoteric oxides? Give two examples of amphoteric oxides.
Answer: Amphoteric oxides are the oxides, which react with both acids and bases to form salt and water. E.g. ZnO and Al2O3.
Question: What types of oxides are formed when non-metals combine with oxygen?
Answer: When non-metals combine with oxygen it forms either neutral or acidic oxides. CO is a neutral oxide; N2O5or N2O3 is an acidic oxide.
Question: Explain why the surface of some metals acquires a dull appearance when exposed to air for a long time.
Answer: This is due to the surface oxidation of metals when exposed to moist air. For e.g. copper turns green on its surface due to the formation of basic copper carbonate Cu(OH) 2. CuCO3. Similarly silver becomes black due to the formation of black Ag2S and Aluminium forms a white coating of Al2O3 on its surface.
Question: Define the following terms.
(i) Minerals
(ii) Ores
(iii) Gangue
Answer: (i) Minerals: All compounds or elements, which occur naturally in the earth’s crust, are called minerals. Example: Alums, K2SO4.Al2(SO4)3 . 24 H2O, Bauxite Al2O3.2H2O
(ii) Ores: Those minerals from which a metal can be profitably extracted are called ores. Bauxite (Al2O3.2H2O) is the ore of Al, copper pyrite CuFeS2. All minerals are not ores but all ores are minerals.
(iii) Gangue: When an ore is mined from the earth, it is always found to be contaminated with sand rocky materials. The impurity of sand and rock materials present in the ore is known as gangue.
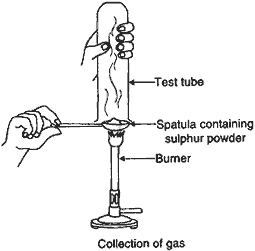
Question: Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test-tube over the burning sulphur.
(a) What will be the action of this gas on:
(i) Dry litmus paper?
(ii) Moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
Answer:
Question: Name two metals, which will displace hydrogen from dilute acids, and two metals which will not.
Answer: Very reactive metals like Zn and Mg displace hydrogen from dilute acids. On the other hand less reactive metals like Cu, Ag, etc. do not displace hydrogen from dilute acids.
Question: In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Answer:
Anode is impure, thick block of metal M.
Cathode is a thin strip/wire of pure metal M.
Electrolyte is a suitable salt solution of metal M.
Question: State two ways to prevent the rusting of iron.
Answer: By coating the surface of iron by rust proof paints. By applying oil or grease to the surface of iron objects so that supply of air consisting of moisture is cut off form the surface.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students