Question: Why is sodium kept immersed in kerosene?
Answer: Sodium metal is kept immersed in kerosene to prevent their reaction with oxygen, moisture and carbon dioxide of air.
Question: Why do ionic compounds have high melting points?
Answer: These compounds are made up of positive and negative ions. There is a strong force of attraction between the oppositively charged ions, so a lot of heat energy is required to break this force of attraction and melt the ionic compounds. This is why ionic compounds have high melting points.
Question: A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Answer: Aqua regia (By volume, this contains 3 parts of concentrated hydrochloric acid and 1 part of concentrated nitric acid) is the solution, which is used to sparkle the bangles like new, but their weight will be reduced drastically.
Question: Write equations for the reactions of
(i) iron with water
(ii) calcium and potassium with water
Answer: (i) Iron reacts with steam to form magnetic oxide of with liberation of H2
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
(ii) Calcium reacts with water to form calcium hydroxide and hydrogen.
Ca(s) + 4H2O(l) → Ca(OH)2(aq) + H2(g)
Potassium reacts with cold water violently immediately with evolution of H2 which catches fire.
2K(s) + 2H2O(l) → 2KOH(aq) + 2H2(g)
Question: What would you observe when zinc is added to a sodium of iron(II) sulphate? Write the chemical reaction that takes place?
Answer: Zinc is more reactive (more electro positive) than iron. Therefore it displaces iron from its salt solution. The colour of ferrous sulphate is pale green which becomes colourless.
FeSO4 + Zn → ZnSO4 + Fe(s)
Light green Zinc sulphate
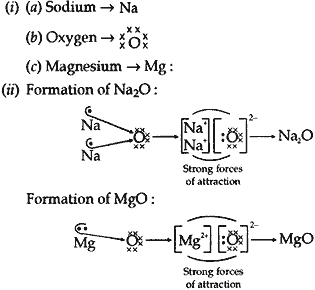
Question: (i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
(iii) Na2O contain Na+ (cation) and O2- (anion) as the ions.
MgO contains Mg2+(cation) and O2- (anion) as the ions.
Question: (a) An are heating in air, gives sulphur dioxide gas. Name the method in each metallurgical step, that will be required to extract this metal from its ore.
(b) State which of the following reactions will take place or not, giving suitable reason for each.
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
Fe(s) + ZnSO4(aq) → FeSO4(aq) + Zn(s)
Answer: (a) As the ore gives SO2 on heating, it is sulphide ore.
Step of metallurgy:
(i) Concentration of ore by froth floatation process.
(ii) Conversion of sulphide ore into oxide by roasting.
(iii) Conversion of metal oxide to metal either by heating alone or by reduction with carbon.
(b) Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
Yes, because Zn is more reactive than copper so will displace copper from copper sulphate solution.
Fe(s) + ZnSO4(aq) → FeSO4(aq) + Zn(s)
No, because Fe is less reactive than Zn.
Question: (a) An are on treatment with dilute hydrochloric acid produces brisk effervescence. What type of ore is this? What steps will be required to obtain metal from the enriched ore?
(b) Copper coin is kept immersed in silver nitrate solution for some time. What change will take place in the coin and colour of the solution? Write the reaction involved.
Answer: (a) Carbonate ore.
Steps are:
1. Calcination: Carbonate ore is heated in limited supply of air and metal oxide is obtained.
2. Reduction with carbon: Oxide ore is heated with carbon.
(b) Copper being more reactive than silver, will displace silver from silver nitrate solution and there will be deposition of silver on copper coin.
The colour of the solution will turn blue.
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + Ag(s)
Question: (1) Define the term alloy. Write two advantage of making alloys.
(2) A metal ‘X’ which is used in thermite process, when heated with oxygen gives an oxide ‘Y’ which is amphoteric in nature. Identify X and Y. Write down the reactions of oxide Y with HCl and NaOH.
Answer:
- Alloy is a homogeneous mixture of two or more metal and a non-metal, mixed in the molten state.
Advantages:
(i) Resistant to corrosion.
(ii) Improved properties of metals. - X is aluminium:
4Al(s) + 3O2(g) → 2Al2O3(s) (Y)
Al2O3 + 6HCl → 2AlCl3 + 3H2
Al2O3 + 2NaOH → 2NaAlO2 + H2O
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students