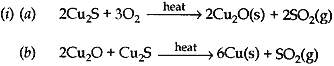Question: (a) Carbon cannot be used as reducing agent to obtain Mg from MgO. Why?
(b) How is sodium obtained from molten sodium chloride? Give equation of the reactions.
(c) How is copper obtained from it sulphide ore? Give equations of the reactions.
Answer: (a) Mg has more affinity for oxygen than for carbon.
(b) Electrolytic reduction process
At cathode: Na+ + e–→ Na
At anode: 2Cl– → Cl2 + 2e–
(c) 2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu2O + Cu2S → 6Cu + SO2
Question: (a) Differentiate between roasting and calcination. Explain the two with the help of suitable chemical equation. How is zinc extracted from its ore?
(b) Name two metals that can be used to reduce metal oxides to metals.
Answer: (a) Calcination:
(i) Heating the ore in limited supply of air.
(ii) Used for carbonate ores.
ZnCO3→ ZnO + CO2
Metal oxide is reduced by using a reducing agent.
ZnO + C → Zn + CO
Roasting:
(i) Heating the ore in excess supply of air.
(ii) Used for sulphide ores.
2ZnS + 3O2 → 2ZnO + 2SO2
(b) Sodium, Calcium, Aluminium
Question: (1). Write the chemical name of the coating that forms on silver and copper articles when these are left exposed to moist air.
(2). Explain what is galvanisation? What purpose is served by it?
(3). Define an alloy. How are alloys prepared? How do the properties of iron change when:
(i) Small quantity of carbon,
(ii) nickel and chromium are mixed with it.
Answer:
- Silver sulphide (Ag2S), copper carbonate (CuCO2).
- Galvanisation is a method of protecting steel and iron from rusting by coating them with a thin layer of zinc. The galvanised article is protected against rusting even if the zinc coating is broken.
- Alloy is a homogeneous mixture of two or more metals or a metal and non-metal. It is prepared by first melting the primary metal and then dissolving the other elements in it indefinite proportion. It is then cooled to room temperature.
(i) On mixing carbon, it becomes hard and strong.
(ii) On mixing Ni, and Cr, it becomes hard and does not rust.
Question: (i) Explain the steps for extraction of copper fro its sulphide ore. Write the balanced equation involved in the process.
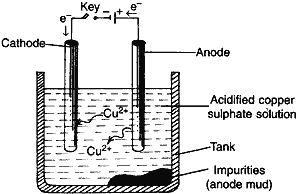
(ii) What is meant by refining of metals? Draw a diagram of electrolytic refining name the substances used as cathode, anode and the electrolyte.
Answer:
(ii) Removal of impurities from the metals after their reduction.
Anode: impure copper
Cathode: pure copper
Electrolyte: acidified CuSO4
Question: (a) List two differences between calcination and roasting in tabular form.
(b) Which method will you use to reduce the following? Explain by giving a suitable example.
(i) Oxides of less reactive metals.
(ii) Oxides of moderately reactive metals.
(iii) Oxides of highly reactive metals.
Answer: (a) Roasting: Heating in presence of excess air. Done for sulphide ores.
Calcination: Heating in limited supply of air. Done for carbonate ores.
(b) (i) Self reduction (In the presence of heat).
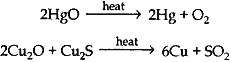
(ii) Reduction using carbon.
ZnO + C → Zn + CO
Sometimes, some highly reactive metals are used as reducing agents.
e.g., 3MnO2 + 4Al → 3Mn + 2Al2O3 + Heat
or Fe2O3 + 2Al → 2Fe + Al2O3 + Heat
(iii) Electrolytic reduction.
e.g., Na, Mg, Ca are obtained by electrolysis of their molten chlorides.
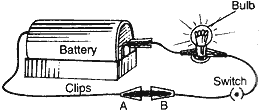
Question: You are given a hammer, a battery, a bulb, wires and a switch.
(1) How could you use them to distinguish between samples of metals and non-metals?
(2) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer:
- Take the given sample of a metal or a non-metal. Beat the sample with hammer and observe. If the given material breaks on beating then it is a non-metal. But if it expands and changes into sheet then it is a metal. Secondly, metals produce sound when they are struck with a hard surface, like hammer, whereas non-metals do not produce any sound
- Taking the help of battery, bulb, wires and switch, set up an electric circuit as shown in the figure. Now, place the metal to be tested in the circuit between terminals A and B as shown in the figure. The bulb will glow if the sample is metal as it can be seen in the figure. The bulb will not glow if the sample is of non-metal.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students